Alginic acid: a highly efficient renewable and heterogeneous biopolymeric catalyst for one-pot synthesis of the Hantzsch 1,4-dihydropyridines†
Mohammad G. Dekamin*a,
Siamand Ilkhanizadeha,
Zahra Latifidoosta,
Hamed Daemib,
Zahra Karimia and
Mehdi Barikanib
aPharmaceutical and Biologically-Active Compounds Research Laboratory, Department of Chemistry, Iran University of Science and Technology, Tehran 16846-13114, Iran. E-mail: mdekamin@iust.ac.ir; Fax: +98 21 77491204
bPolyurethane Department, Iran Polymer and Petrochemical Institute, P. O. Box 14965-115, Tehran, Iran
First published on 27th October 2014
Abstract
Alginic acid, a naturally occurring polysaccharide, in its granular form and without any post-modification was found to be an efficient, environmentally benign, easily recoverable and low-cost catalyst for the clean and rapid synthesis of 1,4-dihydropiridine derivatives (DHPs) just based on its polysaccharide architecture. The Hantzsch pseudo-four-component reaction of ethyl or methyl acetoacetate, ammonium acetate and different aldehydes is catalyzed by alginic acid efficiently under mild conditions to afford the desired products in high to quantitative yields and clean reaction profiles. Avoiding the use of any transition metal, the use of a one-pot and multi-component procedure for the synthesis of DHPs, the reusability of the catalyst and operational simplicity are important features of this methodology.
Design and application of new eco-friendly chemicals and processes have almost been mandatory to keep and increase human health standards due to growing environmental pollution arising from industrial activities and their intensive impact on living systems as well as from an economic point of view.1 Therefore, science and technology have shifted toward green processes nowadays.2 Consequently, developing chemical processes using more environmentally acceptable catalysts, chemicals and atom efficient procedures have emerged as subjects of innovation in green chemistry. For instance, the terrible impact of non-biodegradable wastes is still an open challenge in either developed or developing countries.3 In this regard, great efforts have been performed to use different bio-based feedstocks such as chitosan,4 chitin,5 starch,6 cellulose,7 gelatin,8 wool9 and alginates10 as polymeric supports in the transition metal-based heterogeneous catalytic systems. However, complete replacement of transition metal-based heterogeneous catalytic systems by their metal-free biopolymeric analogues is of significant importance because of both toxicity and difficulty in separation of metal catalysts from the final products, especially in the pharmaceutical industry.11
Alginic acid, also called algin or alginate, is a naturally occurring anionic polysaccharide distributed widely in the cell walls of brown algae. It can also be produced by a microbial fermentation using special bacteria including Azotobacter and Pseudomonas. Alginic acid is an acidic form of alginate and has a plenty of both exposed carboxylic acid and hydroxyl groups inside its backbone. Alginic acid and its salts are linear copolymers with homopolymeric blocks of (1 → 4)-linked α-L-guluronic acid (G) and β-D-mannuronic acid (M) residues (Fig. 1).12 Therefore, it can activate the reaction components by not only Bronsted acid centers but also hydrogen bonding, as a heterogeneous organocatalyst.13 The catalytic activity of alginic acid can also be intensified by its hydrophilic/hygroscopic nature when organic reactions produce water as their byproducts. It is noteworthy that alginic acid is capable of absorbing 200–300 times its own weight in water.4d,10,14 These requirements are fully met in the multicomponent reaction (MCR) for synthesis of the Hantzsch 1,4-dihydropyridines (1,4-DHPs).
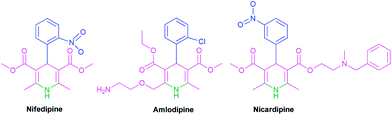
The Hantzsch pseudo-four-component reaction is the oldest and most general known method for the synthesis of 1,4-DHPs and their derivatives which are medicinally and pharmacologically very important molecules. Indeed, commercial drugs such as nifedipine, amlodipine, nicardipine and felodipine constitute a major class of ligands for L-type Ca2+ channels (LTCC) blockers that are widely used to treat conditions such as hypertension and angina (Fig. 2).15 Therefore, the attempts to modify the conditions of the Hantzsch reaction are still of growing importance.
Literature survey shows that several modified methods for Hantzsch reaction have been reported using different catalysts mostly consisting of Bronsted or Lewis acids in recent years.16 For example, homogeneous catalytic systems such as p-TSA,17a 3,4,5-trifluorobenzeneboronic acid in ionic liquid or InCl3,17b Cd(NO3)2·4H2O,17c Yb(OTf)3,17d Sc(OTf)3,17e hafnium(IV) bis(perfluorooctanesulfonyl)imide complex [Hf(NPf2)4]17f and Zn[(L)proline]2,17g can be mentioned. On the other hand, organic Lewis bases such as PPh318a and TMS-protected prolinol18b as well as bifunctional L-proline under ultrasound irradiation18c have also been reported. Furthermore, enzymes such as candida antarctica lipase B19a or baker's yeast,19b ionic liquids,19c and non-ionic surfactant Triton X-10019d demonstrated catalytic activity for DHP synthesis. In spite of their merits, most of these methods suffer from the use of hazardous or expensive catalysts or solvents, long reaction times, high temperature and complicated workup. Although some heterogeneous catalysts such as sulfonic acid supported γ-Fe2O320a or cellulose,20b silica supported 12-tungstophosphoric acid,20c preyssler heteropolyacids,20d Ce(SO4)2–SiO2,20e self-assembled tinphosphonate nanoparticles,20f Zn–VCO3 hydrotalcite,20g and hydromagnesite20h have been introduced in the recent years but there is still room to improve the reaction conditions in terms of the use of renewable biopolymeric and transition metal-free heterogeneous catalysts under mild conditions. In continuation of our interest to explore application of biopolymers in different fields, especially their catalytic activity without any post-modification as well as MCRs,4d,21 we wish herein to report the first catalytic activity of alginic acid (1) as a mild, highly effective and convenient bifunctional heterogeneous biopolymeric catalyst for the one-pot synthesis of 1,4-dihydropyridines via Hantzsch reaction in EtOH (Scheme 1).
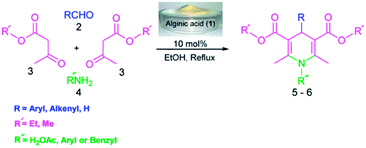 | ||
| Scheme 1 Pseudo-four-component reaction of different aldehydes 2, β-ketoesters 3, and nitrogen sources 4 catalyzed by alginic acid (1) in EtOH under reflux conditions. | ||
To evaluate the catalytic activity of alginic acid (1) for the synthesis of 1,4-DHPs, first the reaction of 4-chlorobenzaldehyde (2a), ethyl acetoacetate (3a), and ammonium acetate (4a) (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 mole ratio) was investigated as the model reaction in EtOH. The results are summarized in Table 1. In the absence of any catalyst, only a poor yield of the corresponding 1,4-DHP 5a was obtained after 4 h (entry 1). Interestingly, the yield of the desired product 5a was significantly improved in shorter reaction times when catalytic amounts of 1 were added to the reaction mixture in EtOH (entries 2–5). The model reaction was also studied in other solvents such as 50% aqueous EtOH (v/v), H2O and EtOAc using alginic acid (1) loading of 10 mol% (entries 6–8). The obtained yield of the desired product 5a in H2O was significantly lower than EtOH under similar catalyst loading and reflux conditions (entries 3 and 6). This may be attributed to both lower solubility of the reactants and rendering the established equilibriums between the reactants and reaction intermediates or desired product 5a to the left side in H2O compared to EtOH (Scheme 4). Finally, the effect of temperature on the obtained yield and required reaction time was also examined (entries 9, 10). The obtained results demonstrated higher yields in shorter reaction times can be obtained in EtOH under reflux conditions.
1.2 mole ratio) was investigated as the model reaction in EtOH. The results are summarized in Table 1. In the absence of any catalyst, only a poor yield of the corresponding 1,4-DHP 5a was obtained after 4 h (entry 1). Interestingly, the yield of the desired product 5a was significantly improved in shorter reaction times when catalytic amounts of 1 were added to the reaction mixture in EtOH (entries 2–5). The model reaction was also studied in other solvents such as 50% aqueous EtOH (v/v), H2O and EtOAc using alginic acid (1) loading of 10 mol% (entries 6–8). The obtained yield of the desired product 5a in H2O was significantly lower than EtOH under similar catalyst loading and reflux conditions (entries 3 and 6). This may be attributed to both lower solubility of the reactants and rendering the established equilibriums between the reactants and reaction intermediates or desired product 5a to the left side in H2O compared to EtOH (Scheme 4). Finally, the effect of temperature on the obtained yield and required reaction time was also examined (entries 9, 10). The obtained results demonstrated higher yields in shorter reaction times can be obtained in EtOH under reflux conditions.
| Entry | Catalyst loading (mol%) | Solvent | Temp. (°C) | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 4-Chlorobenzaldehyde (2a, 1 mmol), ethyl acetoacetate (3a, 2 mmol), ammonium acetate (4a, 1.2 mmol).b Isolated yields.c (50% v/v).d The scale up of model reaction using 4-chlorobenzaldehyde (2a,10 mmol), ethyl acetoacetate (3a, 20 mmol), and ammonium acetate (4a, 11 mmol). | |||||
| 1 | — | EtOH | Reflux | 4 h | 46 |
| 2 | 20 | EtOH | Reflux | 40 | 98 |
| 3 | 15 | EtOH | Reflux | 40 | 98 |
| 4 | 10 | EtOH | Reflux | 50 | 96 |
| 5 | 5 | EtOH | Reflux | 2 h | 63 |
| 6 | 10 | H2O | Reflux | 3 h | 65 |
| 7 | 10 | Aqueous EtOHc | Reflux | 2 h | 77 |
| 8 | 10 | EtOAc | Reflux | 5 h | 62 |
| 9 | 10 | EtOH | 25 | 3.5 h | 89 |
| 10 | 10 | EtOH | 50 | 2 h | 92 |
| 11d | 10 | EtOH | Reflux | 60 | 92 |
Furthermore, the catalytic activity of the alginic acid (1) was examined in the next step to prove its feasibility as a heterogeneous biopolymeric catalyst for scale up of the Hantzsch reaction (entry 11). Interestingly, the alginic acid was found to be an efficient catalyst for performing of the Hantzsch reaction at higher scales. Therefore, alginic acid (1) loading of 10 mol% (17.6 mg per 1 mmol of aldehyde 2) in EtOH under reflux conditions, as the optimized reaction conditions, was developed to other derivatives of aromatic and α,β-unsaturated carbonyl compounds (2b–p) and amines (4a–c) for the synthesis of the desired 1,4-DHPs 5 (Fig. 3 and Scheme 2).
 | ||
| Fig. 3 Different aldehydes (2a–p) and ammonia derivatives (4a–c) examined in the Hantzsch MCR catalyzed by alginic acid (1). | ||
 | ||
| Scheme 2 Pseudo-four-component synthesis of different diethyl 1,4-DHP-3,5-dicarboxylate (5a–r) catalyzed by alginic acid (1) in EtOH under reflux conditions. | ||
To our delight, the diludine (5b), a substance with noteworthy pharmaceutical and food applications as well as organic reductive agent,15,22 was prepared in short reaction time and excellent yield (see also ESI†). Moreover, all the aromatic aldehydes having either electron-withdrawing substitutes (2c–2h) or electron-donating ones (2j–2o) reacted smoothly to provide the corresponding Hantzsch esters (5c–5o) in good to excellent isolated yields. However, the aldehydes carrying nitro groups (2d and 2e) afforded lower yields compared to those having other electron-withdrawing substituents rather than nitro group or even electron-donating ones. Lower yields obtained for aldehydes having meta- and para-nitro substituents (2d and 2e) may be attributed to the competitive formation of less reactive corresponding imines.23 The obtained yields in these cases were improved somewhat by late addition of aldehydes 2d or 2e to the reaction mixture (89% and 84%, respectively). On the other hand, the effect of nitrogen source in the Hantzsch reaction catalyzed by alginic acid (1) was also examined. It has been reported that ammonium salts of carbonate or bicarbonate show somewhat higher efficiency than the acetate salt in hot water.24 However, it should be noted that those former salts produce CO2 on their decomposition as reaction proceeds (Scheme 4). The greenhouse effect of CO2 is well known. Therefore, ammonium acetate is one of the best choice as ammonia source in the Hantzsch reaction. Furthermore, aliphatic or aromatic amines including benzyl amine (4b) and p-toluidine (4c) were also examined. Lower yields of the desired DHPs (5q and 5r, respectively) can be attributed to the more steric hindrance and stronger resonance effect of these amines compared to ammonium acetate.25
In the next stage, the size effect of the alkyl group of ester moiety in the β-ketoester 3 on the yield of the expected 1,4-DHPs was investigated using methyl acetoacetate (3b) instead of ethyl acetoacetate (3a). Corresponding 1,4-DHPs 6a-p were obtained in good to excellent yields (Scheme 3, see also ESI†). In all of studied cases, similar trend of reactivity were observed compared to ethyl acetoacetate (3a).
 | ||
| Scheme 3 Pseudo-four-component synthesis of different dimethyl 1,4-DHP-3,5-dicarboxylate (6a–p) catalyzed by alginic acid (1) in EtOH under reflux conditions. | ||
According to obtained results, the following mechanism can be proposed for the synthesis of 1,4-DHPs 5 and 6 through MCR of aldehydes 2, β-ketoesters 3, and nitrogen sources 4 catalyzed by alginic acid (1) (Scheme 4). First, alginic acid (1) activates the carbonyl functional group of aldehyde 2 for the next addition of enol form of β-ketoesters 3 on it to form the corresponding Knoevenagle intermediate (II). This intermediate is also protonated by alginic acid (1) to be activated for the next Michael addition of enol form of β-ketoesters 3. Then, one of the keto functional groups in the intermediate (IV) is activated through proton transfer from alginic acid (1) to react with the nitrogen source 4 and give imine intermediate (V). This later intermediate is also activated through proton transfer from alginic acid (1) to facilitate its subsequent tautomerization to the corresponding enamine (VI). In the next step, alginic acid (1) activates the remaining keto functional group for ring closure by amino group of the enamine moiety. Finally, elimination of third H2O molecule is catalyzed by alginic acid (1) to afford the desired 1,4-DHP 5 or 6. Furthermore, all the above steps can be competitively catalyzed through hydrogen bonding rather than proton transfer with weaker interactions. On the other hand, ring closure may take place from position 4- of 1,4-DHP rather than its 1-position, alternatively. This means that enamine formation and condensation with the second molecule of β-ketoesters 3 can take place prior to Michael addition in the above proposed mechanism.
 | ||
| Scheme 4 Plausible mechanism for the Hantzsch pseudo-four-component reaction of different aldehydes 2, β-ketoesters 3, and nitrogen sources 4 catalyzed by alginic acid (1). | ||
On the other hand, the reusability of alginic acid (1) was also investigated for at least 6 repeated runs. The results are summarized in Fig. 4. The obtained results demonstrated that alginic acid (1) is reusable for the practical applications in the Hantzsch 1,4-DHPs synthesis.
In conclusion, a new, renewable and readily recoverable biopolymeric catalyst for the MCR Hantzsch reaction has been described which displays particularly high efficiency just based on polysaccharide architecture of alginic acid. Avoiding the use of any transition-metal, clean reaction profiles, the use of a one-pot and multi-component procedure for the synthesis of DHPs, reusability of the catalyst and operational simplicity are the important features of this methodology. Further development of this methodology to bicyclic and tricyclic derivatives of DHPs are currently underway and will be presented in due course.
Acknowledgements
This research was supported by The Research Council of Iran University of Science and Technology (IUST), Tehran, Iran (grant no. 160/1085) and Iran Polymer and Petrochemical Institute, Tehran, Iran.Notes and references
- (a) K. Sanderson, Nature, 2011, 469, 18 CrossRef CAS PubMed; (b) R. A. Sheldon, I. Arends and U. Hanefeld, Green Chemistry and Catalysis, Wiley-VCH, 2007, ch. 1, pp. 1–47 RSC; (c) F. M. Kerton, Y. Liu, K. W. Omaria and K. Hawboldt, Green Chem., 2013, 15, 860 RSC; (d) J. H. Clark, J. Chem. Technol. Biotechnol., 2007, 82, 603 CrossRef CAS; (e) L. Qian and H. Zhang, Green Chem., 2010, 12, 1207 RSC.
- (a) R. A. Sheldon, Chem. Soc. Rev., 2012, 41, 1437 RSC; (b) J. H. Clark, F. E. I. Deswarte and T. J. Farmer, Biofuels, Bioprod. Biorefin., 2009, 3, 72 CrossRef CAS.
- (a) G. Steinhauser and T. M. Klapötke, Angew. Chem., Int. Ed., 2008, 47, 3330 CrossRef CAS PubMed; (b) Y. C. Sharma, B. Singh and J. Korstad, Green Chem., 2011, 13, 2993 RSC; (c) A. Gandini, Green Chem., 2011, 13, 1061 RSC.
- (a) M. Chtchigrovsky, A. Primo, P. Gonzalez, K. Molvinger, M. Robitzer, F. Quignard and F. Taran, Angew. Chem., Int. Ed., 2009, 121, 6030 CrossRef; (b) C. Jouannin, C. Vincent, I. Dez, A. C. Gaumont, T. Vincent and E. Guibal, J. Appl. Polym. Sci., 2013, 128, 3122 CrossRef CAS; (c) R. B. N. Baig and R. S. Varma, Green Chem., 2013, 15, 1839 RSC; (d) M. G. Dekamin, M. Azimoshan and L. Ramezani, Green Chem., 2013, 15, 811 RSC.
- (a) B. M. L. Dioos, I. F. J. Vankelecom and P. A. Jacobs, Adv. Synth. Catal., 2006, 348, 1413 CrossRef CAS; (b) E. S. Medeiros, A. S. F. Santos, A. Dufresne, W. J. Orts and L. H. C. Mattoso, in Polymer Composites, Wiley-VCH, 2013, p. 361 Search PubMed.
- (a) S. Verma, S. L. Jain and B. Sain, ChemCatChem, 2011, 3, 1329 CrossRef CAS; (b) A. Pourjavadi, F. Seidi, S. S. Afjeh, N. Nikoseresht, H. Salimi and N. Nemati, Starch, 2011, 63, 780 CrossRef CAS.
- (a) B. R. Vaddula, A. Saha, R. S. Varma and J. Leazer, Eur. J. Org. Chem., 2012, 34, 6707 CrossRef; (b) H. S. Chandak, N. P. Lad and P. P. Upare, Catal. Lett., 2009, 131, 469 CrossRef CAS; (c) F. Quignard and A. Choplin, Chem. Commun., 2001, 21 RSC.
- X. Zhang, Y. Geng, H. Han, M. Y. Ying, M. U. Huang and Y. Y. Jiang, Polym. Adv. Technol., 2001, 12, 642 CrossRef CAS.
- X. Wang, D. Sui, M. Huang and Y. Jiang, Polym. Adv. Technol., 2006, 17, 163 CrossRef CAS.
- (a) K. R. Reddy, K. Rajgopal and M. L. Kantam, Catal. Lett., 2007, 114, 36 CrossRef CAS; (b) P. L. Boey, S. Ganesan, G. P. Maniam, M. Khairuddean and S. E. Lee, Appl. Catal. A: Gen., 2012, 12, 433 Search PubMed.
- (a) A. Daştan, A. Kulkarnia and B. Török, Green Chem., 2012, 14, 17 RSC; (b) C. J. Li and B. M. Trost, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 13197 CrossRef CAS PubMed.
- (a) K. Y. Lee and D. J. Mooney, Prog. Polym. Sci., 2012, 37, 106 CrossRef CAS PubMed; (b) H. Daemi, M. Barikani and M. Barmar, Carbohydr. Polym., 2013, 92, 490 CrossRef CAS PubMed.
- (a) Z. Du and Z. Shao, Chem. Soc. Rev., 2013, 42, 1337 RSC; (b) P. I. Dalko and L. Moisan, Angew. Chem. Int. Ed., 2004, 43, 5138 CrossRef CAS PubMed; (c) P. R. Schreiner, Chem. Soc. Rev., 2003, 32, 289 RSC; (d) C. Thomas and B. Bibal, Green Chem., 2014, 16, 1687 RSC.
- K. Etzenbach-Effers and A. Berkessel, Noncovalent Organocatalysis Based on Hydrogen Bonding: Elucidation of Reaction Paths by Computational Methods, ed. B. List, Springer-Verlag, Heidelberg, 2010, vol. 291, pp. 1–27 Search PubMed.
- (a) D. J. Triggle, Drug Dev. Res., 2003, 58, 5 CrossRef CAS; (b) J. C. Liang, J. L. Yeh, C. S. Wang, S. F. Liou, C. H. Tsai and I. J. Chen, Bioorg. Med. Chem., 2002, 10, 719 CrossRef CAS; (c) N. Edraki, A. R. Mehdipour, M. Khoshneviszadeh and R. Miri, Drug Discovery Today, 2009, 14, 1058 CrossRef CAS PubMed; (d) M. F. Litvić, M. Litvić, I. Cepanec and V. Vinković, Molecules, 2007, 12, 2546 CrossRef.
- J. P. Wan and Y. Liu, RSC Adv., 2012, 2, 9763 RSC.
- (a) S. R. Cherkupally and R. Mekalan, Chem. Pharm. Bull., 2008, 56, 1002 CrossRef CAS; (b) R. Sridhar and P. T. Perumal, Tetrahedron, 2005, 61, 2465 CrossRef CAS PubMed; (c) R. Tafer, R. Boulcina, B. Carboni and A. Debache, J. Chin. Chem. Soc., 2012, 59, 1555 CrossRef CAS; (d) L. M. Wang, J. Sheng, L. Zhang, J. W. Han, Z. Fan, H. Tian and C. T. Qian, Tetrahedron, 2005, 61, 1539 CrossRef CAS PubMed; (e) J. L. Donelson, R. A. Gibbs and S. K. De, J. Mol. Catal. A: Chem., 2006, 256, 309 CrossRef CAS PubMed; (f) M. Hong, C. Cai and W. B. Yi, J. Fluorine Chem., 2010, 131, 111 CrossRef CAS PubMed; (g) V. Sivamurugan, R. S. Kumar, M. Palanichamy and V. Murugesan, J. Heterocycl. Chem., 2005, 42, 969 CrossRef CAS.
- (a) A. Debache, W. Ghalem, R. Boulcina, A. Belfaitah, S. Rhouati and B. Carboni, Tetrahedron Lett., 2009, 50, 5248 CrossRef CAS PubMed; (b) P. T. Franke, R. L. Johansen, S. Bertelsen and K. A. Jørgensen, Chem.–Asian J., 2008, 3, 216 CrossRef CAS PubMed; (c) S. Guo and Y. Yuan, Chin. J. Chem., 2010, 28, 811 CrossRef CAS.
- (a) J. L. Wang, B. K. Liu, C. Yin, Q. Wu and X. F. Lin, Tetrahedron, 2011, 67, 2689 CrossRef CAS PubMed; (b) J. H. Lee, Tetrahedron Lett., 2005, 46, 7329 CrossRef CAS PubMed; (c) J. S. Yadav, B. V. S. Reddy, A. K. Basak and A. V. Narsaiah, Green Chem., 2003, 5, 60 RSC; (d) P. P. Ghosh, P. Mukherjee and A. R. Das, RSC Adv., 2013, 3, 8220 RSC.
- (a) N. Koukabi, E. Kolvari, M. A. Zolfigol, A. Khazaei, B. S. Shaghasemi and B. Fasahati, Adv. Synth. Catal., 2012, 354, 2001 CrossRef CAS; (b) J. Safari, S. H. Banitaba and S. D. Khalili, J. Mol. Catal. A: Chem., 2011, 335, 46 CrossRef CAS PubMed; (c) E. Rafiee, S. Eavani, S. Rashidzadeh and M. Joshaghani, Inorg. Chim. Acta, 2009, 362, 3555 CrossRef CAS PubMed; (d) A. Gharib, M. Jahangir, M. Roshani and J. W. Scheeren, Synth. Commun., 2012, 42, 3311 CrossRef CAS; (e) W. Pei, Q. Wang, X. Li and L. Sun, Chin. J. Chem., 2010, 28, 483 CrossRef CAS; (f) M. Pramanik and A. Bhaumik, J. Mater. Chem. A, 2013, 1, 11210 RSC; (g) R. Pagadala, S. Maddila, V. D. B. C. Dasireddy and S. B. Jonnalagadda, Catal. Commun., 2014, 45, 148 CrossRef CAS PubMed; (h) U. C. Rajesh, S. Manohar and D. S. Rawat, Adv. Synth. Catal., 2013, 355, 3170 CrossRef CAS.
- (a) M. G. Dekamin and M. Eslami, Green Chem., 2014 10.1039/c4gc00411f; (b) M. G. Dekamin and Z. Mokhtari, Tetrahedron, 2012, 68, 922 CrossRef CAS PubMed; (c) M. G. Dekamin, M. Eslami and A. Maleki, Tetrahedron, 2013, 69, 1074 CrossRef CAS PubMed; (d) H. Daemi, R. R. Rad, M. Barikani and M. Adib, Appl. Catal. A: Gen., 2013, 468, 10 CrossRef CAS PubMed; (e) H. Daemi, M. Barikani and M. Barmar, Int. J. Biol. Macromol., 2014, 66, 212 CrossRef CAS PubMed; (f) H. Daemi, M. Barikani and M. Barmar, Carbohydr. Polym., 2013, 95, 630 CrossRef CAS PubMed; (g) H. Daemi and M. Barikani, Carbohydr. Polym., 2014, 112, 638 CrossRef CAS PubMed; (h) M. G. Dekamin, Z. Mokhtari and Z. Karimi, Sci. Iran., 2011, 18, 1356 CrossRef CAS PubMed.
- (a) J. Stemper, K. Isaac, J. Pastor, G. Frison, P. Retailleau, A. Voituriez, J. F. Betzer and A. Marinetti, Adv. Synth. Catal., 2013, 355, 3613 CrossRef CAS; (b) D. B. Ramachary, M. Kishor and Y. V. Reddy, Eur. J. Org. Chem., 2008, 975 CrossRef CAS.
- (a) A. T. Khan, M. M. Khan and K. K. R. Bannuru, Tetrahedron, 2010, 66, 7762 CrossRef CAS PubMed; (b) J. Li, P. He and C. Yu, Tetrahedron, 2012, 68, 4138 CrossRef CAS PubMed.
- F. Tamaddon, Z. Razmi and A. A. Jafari, Tetrahedron Lett., 2010, 51, 1187 CrossRef CAS PubMed.
- (a) Y. P. Liu, J. M. Liu, X. Wang, T. M. Cheng and R. T. Li, Tetrahedron, 2013, 69, 5242 CrossRef CAS PubMed; (b) S. Balalaie and E. Kowsari, Monatsh. Chem., 2001, 132, 1551 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure, characterization data, IR and 1H NMR spectra of products, and IR spectra and TGA of catalyst. See DOI: 10.1039/c4ra11801d |
| This journal is © The Royal Society of Chemistry 2014 |