Antioxidant properties of several coumarin–chalcone hybrids from theoretical insights†
Abstract
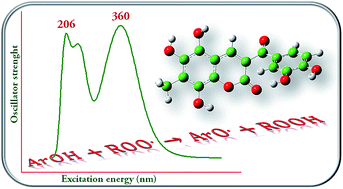
Density functional theory (DFT) and time-dependent formulation of DFT (TDDFT) have been employed to elucidate the structural characteristics, the antioxidant ability, and the UV-Vis absorption properties of a series of coumarin–chalcone derivatives recently synthesized. In addition, to investigate the role of adjacent hydroxyl groups on the antioxidant properties, five additional hybrids were designed and considered in this study. Different antioxidant mechanisms have been investigated. They are hydrogen atom transfer (HAT), electron transfer followed by proton transfer (SET-PT), and sequential proton loss electron transfer (SPLET). Based on the obtained results, the HAT mechanism is proposed as the most important one for the antioxidant protection exerted by this class of compounds. The UV spectra of coumarin–chalcone hybrids are characterized by a band in the region between 300 and 450 nm arising from different electronic transitions. Our investigation confirms the antioxidant properties of these hybrids, and shows that poly-substitution of ring A enhances the antioxidant power of this class of compounds. One of the derivatives, designed in the present work, the 5,6,8-trihydroxy-7-methyl-3-(3′,4′-dihydroxybenzoyl) coumarin, seems to be the most promising candidate as an antioxidant. Accordingly, our calculations strongly encourage the synthesis of coumarin–chalcone hybrids as an important strategy to develop novel compounds with improved antioxidant properties.

 Please wait while we load your content...
Please wait while we load your content...