Effect of alkaline electrolytes on the charge storage capacity and morphology of porous layered double cobalt hydroxide-coated graphene supercapacitor electrodes†
Abstract
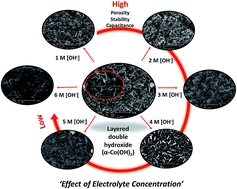
Although alpha-cobalt hydroxide (α-Co(OH)2) with a layered double hydroxide (LDH) structure has been widely used as a supercapacitor electrode, the effect of an alkaline electrolyte on the charge storage performance of the α-Co(OH)2 has not yet been investigated. In this work, α-Co(OH)2 was electrodeposited on reduced graphene oxide-coated carbon fiber paper (rGO/CFP) using chronoamperometry at −0.5 V vs. Ag/AgCl. The effect of alkaline aqueous electrolytes on the performance of the α-Co(OH)2/rGO/CFP electrodes was then investigated by means of scanning and transmission electron microscopies, X-ray photoelectron and absorption spectroscopies, and electrochemical techniques. It was found that the concentrated alkaline electrolytes (i.e., 3–6 M [OH−]) can strip off and/or deform the porous structure of the α-Co(OH)2 deposited on rGO/CFP leading to poor charge storage capacity. 1 M [OH−] was found to be a suitable electrolyte concentration providing high specific capacitance (1096 F g−1 at 1.8 A g−1) without the deformation of the porous α-Co(OH)2 structure after testing. Morphological and electrochemical analyses of the α-Co(OH)2/rGO/CFP electrodes suggest that the effect of the alkaline electrolyte concentration plays a major role on the charge storage performance of α-Co(OH)2-based supercapacitors.

 Please wait while we load your content...
Please wait while we load your content...