Solvent-tuning of ordered mesoporous silicas prepared through evaporation-induced self-assembly templated by poly(ethylene oxide-b-ε-caprolactone)
Abstract
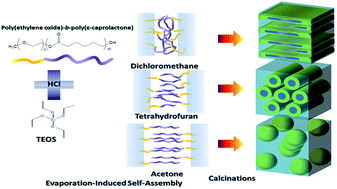
In this study, we employed the diblock copolymer poly(ethylene oxide-b-ε-caprolactone (PEO-b-PCL, EO114CL84) as a template to prepare mesoporous silica materials while controlling the tetraethyl orthosilicate (TEOS)-to-PEO-b-PCL ratio and testing the effects of various solvents [CH2Cl2, tetrahydrofuran (THF), acetone]. Small-angle X-ray scattering, transmission electron microscopy, and N2 adsorption/desorption isotherms revealed that the mesostructures of these mesoporous silicas were influenced by the TEOS content and the solvent. Acetone was superior to CH2Cl2 and THF as the solvent when forming more-ordered mesostructures through evaporation-induced self-assembly (EISA), with the degree of the ordered structure increasing upon increasing the TEOS–to–PEO-b-PCL ratio. In addition, we obtained a long-range-ordered body-centered cubic (bcc) mesoporous structure through the EISA process when the TEOS/PEO-b-PCL ratio was 5 : 1 in acetone; such structures did not form when CH2Cl2 or THF were the solvents. Because the concentration of the template during the EISA process depended on the solvent evaporation rate and solubility, we could prepare highly ordered mesoporous silicas having narrow pore size distributions and various morphologies merely through selection of an appropriate solvent.

 Please wait while we load your content...
Please wait while we load your content...