Wavenumber–intensity joint SERS encoding using silver nanoparticles for tumor cell targeting†
Abstract
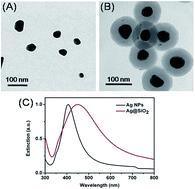
A new optical encoding approach, the wavenumber–intensity joint surface enhanced Raman scattering (SERS) spectral encoding method, was demonstrated by using silver nanoparticles with a core–shell structure. Using three kinds of Raman reporters, 1,4-benzenedithiol (BDT), 2-naphthalenethiol (2-NAT) and 4-methoxythiophenol (4-MT), which were self-assembled on the surfaces of a silver core, 19 codes with distinguished spectral characteristics have been achieved. By conjugating specific antibodies to silver nanoparticles with a certain code, the potential application of such an encoding system in tumor cell targeting has been investigated. The high selectivity of the assay indicates that the joint encoding method could be developed as a powerful tool for high-throughput bioanalysis in the future.

 Please wait while we load your content...
Please wait while we load your content...