Influence of dexamethasone-loaded TNTs on the proliferation and osteogenic differentiation of rat mesenchymal stem cells
Abstract
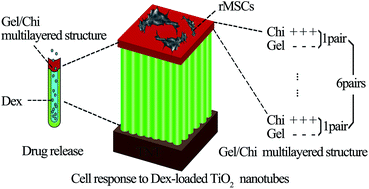
The main cause of titanium implant failure is inferior osseointegration. Dexamethasone (Dex), a potent synthetic member of the glucocorticoid class of steroid drugs, has been widely used due to its potential for inducing osteogenic differentiation of stem cells. In this study, a controlled-release Dex nanocarrier was successfully established by combining titanium nanotubes (TNTs) with self-assembled multilayer gelatin/chitosan (Gel/Chi). The surface characteristics with three different diameter TNTs were investigated by scanning electron microscopy (SEM), contact angle measurement, X-ray fluorescence spectrometer (XRF) and Fourier transform infrared spectrometry (FTIR) and denoted as 30 nm/Dex/(Gel/Chi)6, 70 nm/Dex/(Gel/Chi)6, and 100 nm/Dex/(Gel/Chi)6. During the 14 day drug release assay, Dex released from TNTs demonstrated an initial burst phase followed by a sustained stage. Mesenchymal stem cells isolated from Sprague-Dawley rat bone marrow (rMSCs) and cultured on all the substrate surfaces were observed by confocal laser scanning microscopy (CLSM) and SEM. An MTT assay showed greater cell adherence and proliferation abilities compared with smooth titanium, especially on 70 nm/Dex/(Gel/Chi)6. rMSCs on 30 nm/Dex/(Gel/Chi)6 delineated the highest alkaline phosphatase activity and mineralization levels, as well as gene expression of Runt-related transcription factor 2 (Runx2) and Osterix (OSX) compared with smooth titanium. Above all, the Dex-loaded TNTs were able to promote adherence, proliferation, and osteogenic differentiation of rMSCs. This innovative method may have potential value for constructing titanium implants to accelerate bone integration and improve therapeutic success rates.

 Please wait while we load your content...
Please wait while we load your content...