Circularly polarized luminescence of biaryl atropisomers: subtle but significant structural dependency
Abstract
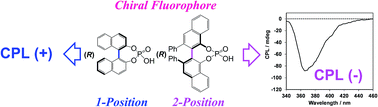
The signs of circularly polarised luminescence and circular dichroism of chiral biaryl fluorophores in the CHCl3-dissolved state (solution state) and the PMMA-film-dispersed state (solid state) were successfully controlled by modifying the internal axial chirality and the axial bonding position of the biaryl units.

 Please wait while we load your content...
Please wait while we load your content...