Reaction mechanism of “amine–borane route” towards Sn, Ni, Pd, Pt nanoparticles†
Abstract
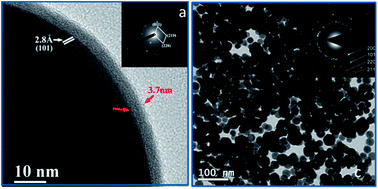
The understanding of the chemical reaction yielding nanomaterials is often based on basic assumptions and novel syntheses are usually presented without knowledge on their reaction mechanism. Herein, the chemical reduction of metal precursors by Lewis complexes of amine–borane is fully investigated, reaction and mechanism, for the synthesis of metallic nanoparticles (NPs). In this study, Sn NPs are obtained by the chemical reduction of tin acetylacetonate complex [Sn(acac)2] using dimethylamine–borane (DMAB) as a reducing agent. The reaction yields single crystalline Sn NPs independently of the use of surfactant. The size of the NPs can be adjusted by regulating the complex/DMAB ratio. The use of DMAB as a reducing agent is extended to the synthesis of Ni, Pd, Pt and Sn/Pd NPs. We investigate the synthetic mechanism by using 11B-NMR and calorimetric analytical tools. The mechanism is found to be specific to the acetylacetonate complexes and involves the reaction of borane on the ligand.

 Please wait while we load your content...
Please wait while we load your content...