A signal-on electrochemiluminescence aptasensor based on the quenching effect of manganese dioxide for sensitive detection of carcinoembryonic antigen†
Ying He,
Yaqin Chai*,
Haijun Wang,
Lijuan Bai and
Ruo Yuan
Education Ministry Key Laboratory on Luminescence and Real-Time Analytical Chemistry, College of Chemistry and Chemical Engineering, Southwest University, Chongqing 400715, People's Republic of China. E-mail: yqchai@swu.edu.cn; yuanruo@swu.edu.cn; Fax: +86-23-68253172; Tel: +86-23-68252277
First published on 27th October 2014
Abstract
We developed a signal-on electrochemiluminescence (ECL) aptasensor by using SI-ATRP to facilitate high-density immobilization of luminophores and manganese dioxide–graphene (MnO2–GO) composite to indirect deactivate the excited state of Ru(dcbpy)32+ for ultrasensitive detection of carcinoembryonic antigen (CEA). In this approach, manganese dioxide–graphene (MnO2–GO) composite served as an efficient quencher for indirect deactivating the excited state of Ru(dcbpy)32+. Surface initiated atom transfer radical polymerization (SI-ATRP) was applied to functionalize multiwalled carbon nanotubes (MWNTs) with glycidyl methacrylate (GMA) as the functional monomer. A nanocomposite material of polyamidoamine (PAMAM) dendrimer encapsulated AuNPs was used as the carrier to combine Ru(dcbpy)32+ and poly-GMA together for the synthesis of the ECL matrices. The prepared matrices were applied to bind amino-modified auxiliary probe I (A1), which was partially complementary with the CEA aptamer. Meanwhile, the MnO2–GO composite was modified with another amino-modified CEA aptamer-partial-complementary auxiliary probe II (A2). Through the hybridization of CEA aptamer with A1 and A2, the quencher MnO2–GO composite was linked with the ECL matrices, by which a low ECL signal was detected (off-state). However, in the presence of CEA, the sandwich-like structure was destroyed because CEA would bind to its aptamer in lieu of the auxiliary probes, which resulted in a recovery of ECL signal (on-state). The proposed ECL aptasensor showed high sensitivity with a detection limit of 25.3 fg mL−1 and a wide linear range of 0.1 pg mL−1 to 20 ng mL−1. Consequently, with the excellent sensitivity, stability and satisfying precision, the as-proposed strategy constitutes a promising detection technique for clinical diagnosis.
Introduction
Electrochemiluminescence (ECL) sensors, especially “quenching effect” based biosensors, have received considerable attention, owing to their intrinsic merits of simple operation, fast response and good regeneration.1,2 It has been found that ferrocene (Fc), dopamine, molecular oxygen, phenols and anilines could significantly quench the ECL emission of Ru(bpy)32+.3–6 Recently, great interest has been focused on semiconductor nanoparticles. MnO2, a well-known transition-metal oxide, is especially attractive for its high theoretical capacity, excellent proton conductivity, environmental compatibility and natural abundance. Most strikingly, Duan and co-workers recently found that amorphous MnO2 could be used as a highly effective water oxidation catalyst to evolve oxygen.7,8 The molecular oxygen can quench the ECL for deactivating the excited state of Ru(dcbpy)32+.9 Therefore, the high oxygen evolution rates of MnO2 can make it as an effective quencher of Ru(dcbpy)32+. To the best of our knowledge, the application of MnO2 in quenching the ECL of Ru(dcbpy)32+ has been studied sparingly in the literature.However, the most of reported biosensors based on the quenching effect are signal-off tape, the high false positive of which limits the wider analytical applications.10,11 The signal-on biosensors based on the quenching effect, as a potential detection methodology, can avoid this disadvantage and will be valuable in both fundamental studies and commercial applications. In order to further improve the sensitivity of this biosensor, some signal amplification strategies have been applied. The Chen group used the polymerase chain reaction (PCR) to increase the immobilization of ECL luminophores.12 The Xiang group proposed a sensitive sensing strategy by integrating the amplification capability of hybridization chain reaction (HCR).13 However, PCR and HCR are always harsh terms, limited to the inefficient enzyme and sophisticated equipment. Surface initiated atom transfer radical polymerization (SI-ATRP) has been proposed as a potential “grafting-from” method to achieve both high stability of polymer layer and high graft density for surface modification.14,15 The advantages of SI-ATRP for the synthesis of new polymers with optical properties have made this technique very popular in recent years. Lei and co-workers prepared the SI-ATRP by functionalizing MWNTs with polyethyleneimine (PEI)16 which ensured the good separation and stability of MWNTs due to the electrostatic interactions in aqueous solution. Glycidyl methacrylate (GMA), a well-used SI-ATRP monomer, can be used to graft as the polymer incorporating a large number of epoxy groups on the surface which could further react with various functional groups (amino, hydroxyl, carboxyl etc.).17 Meanwhile, PAMAM has been used as carrier of affinity ligands due to their unique properties, such as good water-solubility, chemical stability, and easily functionalized.18,19 All these above provide a new viewpoint for us to synthesize a novel ECL matrices through combining SI-ATRP with Ru(bpy)32+–pendant PAMAM.
Herein, we described a signal-on CEA aptasensor based on the quenching effect of manganese dioxide–graphene (MnO2–GO) composite to the ECL of Ru(dcbpy)32+. In this construction, an innovational SI-ATRP approach was used to functionalize MWNTs by pretreating the MWNTs with PEI and using GMA as the monomer to in situ grow the epoxy groups riched noncross-linked polymer chains from the sidewalls of the MWNTs. And amino-terminated PAMAM encapsulated AuNPs was modified with Ru(dcbpy)32+ to form Ru(dcbpy)32+–pendant PAMAM. Owing to the remaining amine group, Ru(dcbpy)32+–pendant PAMAM reacted with the epoxy groups on the poly-GMA to form the ECL matrices (Ru–pendant-PHMWNT). The formed matrices achieved a high ECL signal for the large amount of immobilized Ru(dcbpy)32+. Next, we used a facial and efficient method to synthesize MnO2–GO composite. The designed MnO2–GO composite possess a heavy loading capacity of MnO2 and a large active surface area. Then the amino-modified CEA aptamer-partial-complementary A1 was immobilized on the ECL matrices via the interaction between AuNPs and amino group on A1. Meanwhile, MnO2–GO composite was modified with another amino-modified CEA aptamer-partial-complementary A2. Through the hybridization of CEA aptamer and its partial complementary A1 and A2, the ECL matrices (Ru–pendant-PHMWNT) and the quencher (MnO2–GO composite) linked together. The ECL of Ru(dcbpy)32+ can be effectively. Therefore, a low ECL signal was detected in such a state. In the presence of CEA, the CEA aptamer was released for the reason that the CEA aptamer preferred to form the CEA–aptamer complex in lieu of the aptamer–DNA duplex20 and the quencher (MnO2–GO composite) departed away from the ECL matrices, leading to a recovery of ECL signal. Accordingly, the CEA could be detected quantitatively through the variation in ECL intensity.
Experimental
Reagents
Carcinoembryonic antigen (CEA), prostate specific antigen (PSA) and alpha-fetoprotein antigen (AFP) were received from Biocell (Zhengzhou, China). Tris(4,4′-dicarboxylicacid-2,2′-bipyridyl)ruthenium(II)dichloride (Ru(dcbpy)32+), bovine serum albumin (BSA, 96–99%), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), hemoglobin (Hb), N-hydroxy succinimide (NHS) and HAuCl4·4H2O were purchased from Sigma-Aldrich (St. Louis, MO, USA). Poly (ethylenimine) (PEI, 50%) was purchased from Fluka (Switzerland). Polyamidoamine (PAMAM) dendrimer was purchased from Weihai CY Dendrimer Technology Co., Ltd (Weihai, China). 2-Bromo-2-methylpropionyl bromide (BIB, 98%), triethylamine (TEA, 99%), hexanethiol (HT), oleic acid, KMnO4, glycidyl methacrylate (GMA, 96%) and 1,1,4,7,7-pentamethyldiethylenetriamine (98%) were received from J&K Scientific Ltd (China). Graphene oxide (GO) was purchased from Nanjing XFNANO Materials Tech Co., Ltd (Nanjing, China). Multiwall carbon nanotubes (MWNTs) (>95% purity) were purchased from Chengdu Organic Chemicals Co., Ltd. of the Chinese Academy of Science (Chengdu, China). Methanol and ethanol were purchased from Chengdu KeLong Chemical Co., Ltd (Chengdu, China). Tetrahydrofuran (THF) and toluene were purchased from Chongqing Chuandong Chemical (Group) Co., Ltd (Chongqing, China). Nafion was diluted to 1 wt% with ethanol solution. Tris(hydroxymethyl) aminomethane (TRIS) was purchased from Roche (Switzerland). Phosphated buffered solutions (PBS, pH 7.4, 0.1 M) were prepared using 0.1 M Na2HPO4, 0.1 M KH2PO4 and 0.1 M KCl. Ferricyanide solutions (Fe(CN)63−/4−, 5.0 mM, pH 7.4) were obtained by dissolving potassium ferricyanide and potassium ferrocyanide with PBS (pH 7.4).Auxiliary probe I (A1): 5′-NH2-AAAAAATTGAA-3′
Auxiliary probe II (A2): 5′-CTGGTATAAAA-NH2-3′
CEA aptamer: 5′-ATACCAGCTTATTCAATT-3′
were purchased from TaKaRa (Dalian, China). The human serum samples were provided by Da Ping Hospital (Chongqing, China). All chemicals were of analytical grade and used as received. The doubly distilled water used was obtained from a Milli-Q water purifying system (18 MΩ cm).
Apparatus
MPI-A ECL analyzer (Xi'an Remax Electronic Science & Technology Co. Ltd., Xi'an, China) was used to measure the ECL signals with the voltage of the photomultiplier tube (PMT) set at 800 V in the process of detection. Cyclic voltammetric (CV) and electrochemical impedance spectroscopic (EIS) measurements were carried out with a CHI 600d electrochemistry workstation (Shanghai CH Instruments, China). A three-electrode system was employed with an Ag/AgCl (sat. KCl) reference electrode, a platinum wire counter electrode and bare or modified glassy carbon electrode (GCE) as working electrode, respectively. The morphologies of nanoparticles were estimated using a scanning electron microscope (SEM, S-4800, Hitachi Instrument, Japan). X-ray photoelectron spectroscopy (XPS) measurements were carried out using a VG Scientific ESCALAB 250 spectrometer (Thermoelectricity Instruments, USA) and using Al Kα X-ray (1486.6 eV) as the light source.Preparation of non-cross-linked polymer chains hybrid MWNTs (PHMWNT)
The amine-functionalized MWNTs were prepared through the electrostatic interactions between MWNTs and PEI solution according to the literature with a little modification.21 Briefly, MWNTs (5.0 mg) were dispersed in 2 wt% PEI salt solution (0.5 M NaCl, 20 mL) with the help of ultrasonic treatment for 3 h to give a homogeneous black suspension, then stirred overnight at 80 °C, and again placed in an ultrasonic bath for 30 min. The amine-functionalized MWNTs (MWNTs–PEI) were collected by repeated centrifugation and redispersion in 0.1 M PBS (pH 7.4) and ethanol twice respectively.To synthesize MWNTs-supported initiator, the MWNTs–PEI were suspended in pre-cooled THF–toluene (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, containing 55 mM TEA), then BIB was added dropwisely with vigorous stirring (final concentration, 50 mM). The reaction was carried out for 15 min at 0 °C and then at RT for another 2 h. The excess initiators were removed by repeated washing with methanol and SI-ATRP grafting was carried out by adding nitrogen purged mixture of 2 M GMA, 0.01 M CuCl, 0.001 M CuCl2, and 0.015 M 1,1,4,7,7-pentamethyldiethylenetriamine to the initiator immobilized MWNTs solution, sealed and agitated at 60 °C for 4–6 h. After reaction, excess reagents were removed by repeated washing with methanol. Then the non-cross-linked Polymer Chains Hybrid MWNTs (PHMWNT) was obtained.
1, v/v, containing 55 mM TEA), then BIB was added dropwisely with vigorous stirring (final concentration, 50 mM). The reaction was carried out for 15 min at 0 °C and then at RT for another 2 h. The excess initiators were removed by repeated washing with methanol and SI-ATRP grafting was carried out by adding nitrogen purged mixture of 2 M GMA, 0.01 M CuCl, 0.001 M CuCl2, and 0.015 M 1,1,4,7,7-pentamethyldiethylenetriamine to the initiator immobilized MWNTs solution, sealed and agitated at 60 °C for 4–6 h. After reaction, excess reagents were removed by repeated washing with methanol. Then the non-cross-linked Polymer Chains Hybrid MWNTs (PHMWNT) was obtained.
Preparation of Ru(dcbpy)32+–pendant PAMAM modified polymer chains hybrid MWNTs (Ru–pendant-PHMWNT)
In brief, 1 mM HAuCl4 was added to 0.07 mM PAMAM under continual stirring and kept in the dark for 1 h at RT. In doing so, AuCl4− were partitioned into the nitrogen-rich interior of the PAMAM forming PAMAM–Au(III) complex. Next, 20 mM NaBH4 was quickly added to PAMAM–Au(III) solution with vigorous stirring. The color of the resulting mixed solution immediately turned from light yellow to red-brown with the reducing of Au(III) to Au (0) and forming PAMAM–Au. Excess PAMAM and NaBH4 were removed by centrifuging, and the precipitates were washed twice with 0.1 M PBS (pH 7.4) and stored at 4 °C for further use.40 mg EDC and 10 mg NHS were successively added into 2 mL 6.45 μM Ru(dcbpy)32+ solution to activate the carboxyl groups of Ru(dcbpy)32+ for 2 h. Followed that, PAMAM–Au was added into the solution under continuous stirring for about 8 h. Then the prepared PHMWNT was redispersed in the above mixed solution. The resulting mixture was reacted for 12 h at RT under gentle shaking. After that the Ru–pendant-PHMWNT was obtained by centrifuging and washing several times with 0.1 M PBS (pH 7.4).
Preparation of MnO2–GO–A2
The MnO2–GO composite was synthesized by using an in situ replacement process to oxidize part of the carbon atoms in the framework of the GO.22 Firstly, the GO (1.0 mg mL−1) was dispersed in 0.1 M PBS (pH 7.4) by means of ultrasonic treatment for 0.5 h, while different concentrations of KMnO4 solution were added rapidly. The mixture was kept in the dark for 12 h at RT. The purple color of the solution turned to brown gradually and no obvious precipitation occurred during the reaction. Finally, the MnO2–GO composite was centrifuged, washed, and redispersed in 0.1 M PBS (pH 7.4).And then 0.5 mL EDC and NHS (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixed solution was added to the above solution and stirred for 2 h. Subsequently, A2 (2.5 μM, 200 μL) was injected into the mixture and stirred for overnight to form the amide bond between the carboxyl of MnO2–GO composite and the amino of A2. After that the as-prepared MnO2–GO–A2 were washed for several times to remove free A2, redispersed in 1 mL 0.1 M PBS (pH 7.4) and stored at 4 °C.
1) mixed solution was added to the above solution and stirred for 2 h. Subsequently, A2 (2.5 μM, 200 μL) was injected into the mixture and stirred for overnight to form the amide bond between the carboxyl of MnO2–GO composite and the amino of A2. After that the as-prepared MnO2–GO–A2 were washed for several times to remove free A2, redispersed in 1 mL 0.1 M PBS (pH 7.4) and stored at 4 °C.
Preparation of A1–CEA aptamer double-stranded DNA (A1–CEA aptamer-dsDNA)
5 μL of A1 (100 μM) and 5 μL of CEA aptamer (100 μM) were diluted to 5 μM with Tris–HCl buffer (pH 7.4), respectively. Then the above solution was mixed under slow stirring for about 2 h at 4 °C to form A1–CEA aptamer-dsDNA. Finally, the obtained A1–CEA aptamer-dsDNA was stored at 4 °C for further use.Preparation of the aptasensor
Scheme 1 showed the schematic diagram of preparation of the ECL aptasensor. This involved coating Nafion on the electrode, dropping Ru–pendant-PHMWNT on the Nafion modified electrode via the electrostatic interaction, self-assembly of the A1–CEA aptamer-dsDNA on the electrode surface, surface blocking with HT, association of MnO2–GO–A2. Then the proposed aptasensor was incubated with 15 μL CEA standard solutions at different concentrations for 40 min at room temperature. Finally, the aptasensor was washed and investigated with a MPI-A ECL analyzer in 3 mL 0.1 M PBS (pH 7.4) at RT. | ||
| Scheme 1 Schematic illustration of (A) preparation of ECL matrices (Ru–pendant-PHMWNT) and (B) detection principle of the CEA aptasensor via the signal-on ECL methodology. | ||
Results and discussion
Characterization of different nanomaterials
The morphologies of different nanomaterials were characterized by scanning electron microscopy (SEM) at an acceleration voltage of 20 kV. Compared with the well-dispersed tube-like structure of MWNTs (Fig. 1(A), inset), the surface of Ru–pendant-PHMWNT became more round and bright (Fig. 1(A)), which indicated that MWNTs had been surrounded by an organic polymer layer and there were some conducting material on the surface. As confirmed by XPS, the characteristic peaks for N (A), O (A), Au 4p, Ru 3p, N 1s, C 1s, and core level regions could be obviously observed at the Ru–pendant-PHMWNT in Fig. 1(B). Therefore, it was reasonable to conclude that the poly-GMA was grafted onto the MWNTs surface and the Ru–pendant-PHMWNT was successfully prepared. | ||
| Fig. 1 (A) SEM images of Ru–pendant-PHMWNT. Insert in A: SEM images of MWNTs. (B) XPS analysis of Ru–pendant-PHMWNT. | ||
Optimization of detection conditions
The performance of the aptasensor was closely related to two factors. One was the proportion of MnO2–GO composite, the other was the incubation time of MnO2–GO–A2. To investigate the effect of the proportion of MnO2–GO composite on the ECL quenching of Ru(dcbpy)32+, different concentrations of KMnO4 were added into GO (1.0 mg mL−1) solution to synthesize the MnO2–GO composite in various proportions. As shown in Fig. 2, the ECL intensities sharply decreased with the increase of KMnO4 concentration until a limiting quenching coefficient of 80% was reached. Therefore, a KMnO4 concentration of 50 mM was used in subsequent experiments. The incubation time of MnO2–GO–A2 could affect the hybridization between A2 and CEA aptamer, which influenced the ECL of this system accordingly. As shown in Fig. 3(A), the sufficient quenching of Ru(dcbpy)32+ by MnO2–GO need a relatively minimum incubation time of 30 min to observe maximum ECL quenching. A time of 30 min was subsequently adopted in this study.In addition, another significant factor that can impact the aptasensor for the recovery of ECL is the CEA incubation time. As shown in Fig. 3(B), the ECL intensity increased rapidly upon increasing the incubation time to 40 min, and longer incubation time did not enhance the response further. Therefore, 40 min was chosen as the incubation time for the detection of CEA.
Characteristics of the ECL aptasensor
ECL signals at each immobilization steps were recorded in 0.1 M PBS (pH 7.4) to monitor the fabrication of the aptasensor. The voltage of the photomultiplier tube (PMT) was set at 800 V and the applied potential was 0.2–1.25 V (vs. Ag/AgCl) with a scan rate of 100 mV s−1 in the process of detection. The corresponding ECL results were shown in Fig. 4(A). First, almost no ECL signal was observed on the bare GCE (curve a) and GCE/Nafion (curve b), which could be attributed to the lack of luminescence reagent. When Ru–pendant-PHMWNT complex was coated on the modified electrode, a quite high ECL signal was observed (curve c). After the A1–CEA aptamer-dsDNA was successfully immobilized onto the electrode through the interaction between AuNPs and amino groups of A1, a decreased ECL signal was obtained (curve d). The reason was that A1–CEA aptamer-dsDNA obstructed the electron transport. Followed by employing HT to block nonspecific binding sites, a successive decline of ECL signal was detected (curve e). Moreover, when the MnO2–GO–A2 hybridized with the CEA aptamer on the electrode surface, a large decrease of ECL signal was obtained (curve f). This confirmed that the ECL of Ru(dcbpy)32+ can be effectively quenched by MnO2–GO composite. Finally, after incubating with CEA, a large increase of ECL signal was observed (curve g), demonstrating that the CEA aptamer preferred to form the CEA–aptamer complex in lieu of the aptamer–DNA duplex.The fabrication process of the ECL aptasensor was also characterized by cyclic voltammograms (CVs) in 5 mM [Fe(CN)6]3−/4− with a scanning potential from −0.2 to 0.6 V and at a scan rate of 100 mV s−1. A well-defined redox peaks of [Fe(CN)6]3−/4− was obtained at bare GCE (Fig. 4(B), curve a).
When Nafion was coated on the GCE, the peak current of the modified electrode markedly decreased due to Nafion strongly obstructed the electron transport (curve b). Then Ru–pendant-PHMWNT complex was modified on GCE/Nafion, and the peak current increased greatly (curve c) for the reason that MWNTs and AuNPs could largely facilitated electron transmission. Subsequently, the peak decreased gradually with the immobilizing of A1–CEA aptamer-dsDNA (curve d), which suggested the successful modification of A1–CEA aptamer-dsDNA and the increase of the steric hindrance. After incubating with HT, the decreased peak current was also observed (curve e). Finally, after the MnO2–GO–A2 were coupled with the CEA aptamer of the electrode, a small decrease of the current could be observed (curve f), accounting for the binding between A2 and CEA aptamer which retard the electron transfer tunnel.
ECL detection of CEA with aptasensor
To assess the sensitivity and the quantitative range of the proposed ECL aptasensor, the prepared aptasensor was subjected to solutions with different concentration of CEA under the optimal experimental conditions. As expected, the intensity of the recovery ECL enhanced gradually with the increasing concentration of CEA (Fig. 5 curves a–h). There was a good linear relationship between ECL intensity and the logarithm of CEA concentrations ranged from 0.1 pg mL−1 to 20 ng mL−1 with a limit detection of 25.3 fg mL−1. The regression equation was I = 1084.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cCEA + 6622.5 with a correlation coefficient of 0.9971 (where I was the ECL intensity (a.u.), and cCEA was the concentration of CEA).
cCEA + 6622.5 with a correlation coefficient of 0.9971 (where I was the ECL intensity (a.u.), and cCEA was the concentration of CEA).
Stability, selectivity and reproducibility of the aptasensor
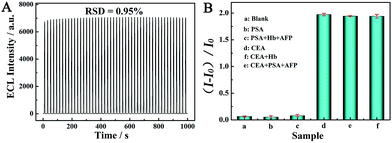
The ECL intensities of the aptasensor remained at a comparatively stable value (0.95% variation) during consecutive cyclic potential scanning for 48 cycles (Fig. 6(A)), indicating a good stability for ECL detection. The good stability of the aptasensor may be originated from the excellent long-term stability of Ru–pendant-PHMWNT and the good bioactivity and catalytic of MnO2–GO composite.To evaluate the specificity of the detection method, the aptasensor was incubated with 1 ng mL−1 CEA containing different interferents such as Hb, AFP, PSA, and also incubated with 20 ng mL−1 PSA, AFP, Hb solution. By comparing the values of (I − I0)/I0 from the different proteins, (I and I0 correspond to the ECL intensity of the resulting aptasensor after and before adding targeted protein), CEA can be clearly distinguished from other proteins (Fig. 6(B)). Such results indicated that the proposed aptasensor had good selectivity.
To estimate the reproducibility of the proposed aptasensor, the intra-assay imprecision of five equally prepared aptasensors in the same batch and inter-assay imprecision at five aptasensors made at the same electrode in batches for detection of 1 ng mL−1 CEA were examined. The relative standard deviation (RSD) of the intra-assay was 1.2%, and the inter-assay precision was 1.3%, indicating that the reproducibility of the proposed aptasensor was satisfying.
Detection of CEA in clinical serum samples and evaluation of method trueness
In order to evaluate the feasibility of the proposed immunosensor for clinical applications, the aptasensor was used for the determination of CEA by standard addition methods in serum samples. The experiment was performed by spiking various levels of CEA standards into normal human sera which were diluted with 0.1 M PBS (pH 7.4) to suitable concentration. The results showed satisfactory recoveries in the range of 92.13–108.3% (Table 1), which provided a promising alternative tool for determination of proteins in clinic analysis.| Sample number | Added (ng mL−1) | Found (ng mL−1) | Recovery (%) |
|---|---|---|---|
| 1 | 0.001 | 0.0009802 | 98.02 |
| 2 | 0.01 | 0.009421 | 94.21 |
| 3 | 0.1 | 0.09213 | 92.13 |
| 4 | 4.0 | 3.943 | 98.58 |
| 5 | 20.0 | 21.65 | 108.3 |
Conclusions
In this work, we proposed a new signal-on aptasensor based on the quenching effect of MnO2–GO composite to the ECL of Ru(dcbpy)32+ for ultrasensitive detection of CEA. First, the ECL matrices Ru–pendant-PHMWNT achieved a strong ECL signal through the use of SI-ATRP. Next, due to the synergistic contribution of two functional components, MnO2–GO composite served as an effective quencher of Ru(dcbpy)32+. Overall, the proposed aptasensor was of great potential for detection of CEA due to the high sensitivity, good stability, and satisfying selectivity.Acknowledgements
This work was financially supported by the NNSF of China (21275119, 21075100), Ministry of Education of China (Project 708073), Natural Science Foundation of Chongqing City (CSTC-2011BA7003), Specialized Research Fund for the Doctoral Program of Higher Education (20100182110015) and the Postgraduate Science and Technology Innovation Program of Southwest China University (Grant no. XDJK2012A004, XDJK2013A008).Notes and references
- M. Kaneko and H. Nakamura, Macromolecule, 1987, 20, 2265 CrossRef CAS.
- Q. Lu, W. Wei, Z. X. Zhou, Z. X. Zhou, Y. J. Zhang and S. Q. Liu, Analyst, 2014, 139, 2404 RSC.
- X. Q. Lu, H. F. Wang, J. Du, B. M. Huang, D. Liu, X. H. Liu, H. X. Guo and Z. H. Xue, Analyst, 2012, 137, 1416 RSC.
- M. S. Wu, D. J. Yuan, J. J. Xu and H. Y. Chen, Anal. Chem., 2013, 85, 11960 CrossRef CAS PubMed.
- J. McCall, C. Alexander and M. Richter, Anal. Chem., 1999, 71, 2523 CrossRef CAS PubMed.
- H. Zheng and Y. Zu, J. Phys. Chem. B, 2005, 109, 16047 CrossRef CAS PubMed.
- F. M. M. Najafpour, M. Kompany-Zareh, A. Zahraei, D. J. Sedigh, H. Jaccard, M. Khoshkam, R. D. Britt and W. H. Casey, Dalton Trans., 2013, 42, 14603 RSC.
- L. L. Duan, L. P. Tong, Y. H. Xu and L. C. Sun, Energy Environ. Sci., 2011, 4, 3296 CAS.
- Y. N. Cheng, J. Yang, Y. Qu and P. X. Li, Org. Lett., 2012, 14, 98 CrossRef CAS PubMed.
- Y. Xiao, B. D. Piorek, K. W. Plaxco and A. J. Heeger, J. Am. Chem. Soc., 2005, 127, 17990 CrossRef CAS PubMed.
- B. L. Li, Y. Jiang, X. Chen and A. D. Ellington, J. Am. Chem. Soc., 2012, 134, 13918 CrossRef CAS PubMed.
- L. H. Guo, H. H. Yang, B. Qiu, X. Y. Xiao, L. L. Xue, D. Kim and G. N. Chen, Anal. Chem., 2009, 81, 9578 CrossRef CAS PubMed.
- Y. Chen, M. L. Yang, Y. Xiang, R. Yuan and Y. Q. Chai, Nanoscale, 2014, 6, 1099 RSC.
- Y. Zhou, S. X. Wang, B. J. Ding and Z. M. Yang, Chem. Eng. J., 2008, 138, 578 CrossRef CAS PubMed.
- G. Huang, Z. Sun, H. Q. Qin, L. Zhao, Z. C. Xiong, X. J. Peng, J. J. Ou and H. F. Zou, Analyst, 2014, 139, 2199 RSC.
- Z. L. Lei, X. Y. Wei, L. Zhang and S. X. Bi, Colloids Surf., A, 2008, 324, 131 CrossRef CAS PubMed.
- J. H. Liu, N. Feng, S. Q. Chang and H. L. Kang, Appl. Surf. Sci., 2012, 258, 6127 CrossRef CAS PubMed.
- J. J. Chen, X. Y. Cao, R. Guo, M. W. Shen, C. Peng, T. Y. Xiao and X. Y. Shi, Analyst, 2012, 137, 223 RSC.
- K. Takada and H. D. Abruna, J. Electroanal. Chem., 2004, 567, 249 CrossRef CAS PubMed.
- C. L. Smith, US Pat. Appl. Publ., US2010/0254901 A1, 7 Oct 2010.
- L. Q. Jiang and L. Gao, Carbon, 2003, 41, 2923 CrossRef CAS.
- Y. Zhang, M. Su, L. Ge, S. G. Ge, J. H. Yu and X. R. Song, Carbon, 2013, 57, 22 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra11392f |
| This journal is © The Royal Society of Chemistry 2014 |