Stereoselective adsorption utilizing L-phenylalanine imprinting chiral ordered mesoporous silica†
Meng-xiang Suab,
Zhong-ying Liuc,
Jin-long Chen*ab,
Li-fei Chengd,
Bo Liab,
Fang Yanab and
Bin Di*ab
aDepartment of Pharmaceutical Analysis, China Pharmaceutical University, Nanjing 210009, China. E-mail: dibin@cpu.edu.cn; chenjl_4@hotmail.com; Fax: +86 2583271269; Tel: +86 2583271269
bKey Laboratory of Drug Quality Control and Pharmacovigilance (China Pharmaceutical University), Ministry of Education, Nanjing 210009, China
cSichuan Institute for Food and Drug Control, Chengdu 611731, China
dDepartment of Pharmacy, Kouqiang Hospital, Fourth Military Medical University, Xi'an 710032, China
First published on 20th October 2014
Abstract
L-Phenylalanine imprinting chiral ordered mesoporous silica (L-Phe-COMS) was facilely synthesized in the presence of phenylalanine amino acid by combining tetraethyl orthosilicate and quaternized aminosilane silica sources. The obtained COMS was comparable with a MCM-41-type structure, with narrow pore size distribution, and high specific surface area characterized by powder X-ray diffraction and N2 adsorption experiments. The imprinting chirality of COMS was disclosed by mixed and separate L- and D-phenylalanine adsorption on the L-Phe-COMS with a stereoselective adsorption capacity of up to 3.24. In addition, six racemic mixtures including amino acids and drugs were explored to test the stereoselective adsorption capacity of L-Phe-COMS. The imprinting chiral ordered mesoporous silica presents the advantages of straightforward synthesis approach and robust stereoselective adsorption capacity, making it a promising candidate for chiral adsorption and separation.
Introduction
Recently, chiral ordered mesoporous silica (COMS) materials have become the centre of attention and have provided a new approach to obtain pure enantiomers because of their high surface area, large pore volume, decorating accessibility of the pore wall or the framework and high thermo stability.1–4 Currently, COMS are mainly synthesized from an achiral silica source, by combining chiral surfactants or achiral surfactants.5–7 COMS materials have also been previously developed in our laboratory,8 which were synthesized using the chiral anionic surfactant as a single template. However, there are some defects in the synthesis using chiral surfactants, for example, most of the chiral surfactants are difficult to synthesize, sometimes even multiple surfactants or chiral molecules are needed as inducers in the procedure.Amino acids are pH-sensitive zwitterionic surfactants used as inducers, which display the properties of anionic compounds at high pH. Yokoi et al. demonstrated that a simple amino acid monomer can promote the formation of silica, resulting in the preparation of well-ordered silica nanospheres.9 Furthermore, with their simple structures and the ready availability of both enantiomers, amino acids provide an inexpensive approach for resolution studies and have been used either as the racemate for resolution or as the chiral selector for the resolution of racemic mixtures of several other compounds.10 Coronas et al. reported the COMS imprinting with amino acids arginine, histidine, isoleucine, and proline exhibiting enantioselectivity.11,12 Moreover, L-phenylalanine (L-Phe) has been successfully applied to chiral chromatographic separation as chiral selector in the mobile phase.13–15 Moreover, phenylalanine is a well-known α-amino acid that is essentially possessed by all humans in the L-configuration. Phenylalanine, contains benzene, amino and carboxylic acid moieties, which facilitates the formation of COMS through non-covalent multiple interactions, such as electrostatic and hydrophobic interactions, with silica resources and surfactants added.16,17
However, to date, there have been few reports to utilize the COMS imprinting with L-Phe in stereoselective adsorption. In this work, L-Phe imprinting COMS (L-Phe-COMS) was synthesized in basic media by combining silica sources tetraethyl orthosilicate and quaternized aminosilane (in a templating reagent role) together with the L-phenylalanine amino acid. L-Phe-COMS showed a stereoselective adsorption capacity of up to 3.24 for D,L-phenylalanine. The possible mechanisms of synthesis and the stereoselective adsorption were also discussed in this work.
Experimental
Synthesis of L-phenylalanine imprinting chiral ordered mesoporous silica
L-Phe-COMS were generally prepared using tetraethyl orthosilicate (TEOS) as the main silica source and the N-3-[3-(trimethoxysilyl) propyl]-N-octadecyl-N,N-dimethylammonium chloride (C18-TMS) as the initiator, with the molar composition of TEOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C18-TMS
C18-TMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-phenylalanine:H2O
L-phenylalanine:H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaOH = 6
NaOH = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1:2
1:2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000
1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. Typically, 0.605 g of L-phenylalanine was dissolved in 47.58 mL ultrapure water at constant temperature, and then 2.637 g C18-TMS, 3.337 g TEOS were added stepwise and the mixture was stirred at a constant temperature. The apparent pH value was about 11 until the end of the synthesis. The resulting mixtures were poured into a Teflon kettle and maintained at 80 °C for 24 h. Then, the product was washed with deionized water several times till the pH value decreased to approximately 7.0, washed with ethanol three times, centrifuged at 3000 rpm, and the precipitate was dried overnight at 80 °C. Finally, the COMS material was obtained after calcination at 650 °C for 8 h to remove the organic materials. For comparison purposes, samples were also prepared without L-phenylalanine (blank COMS). The major material source is described in the ESI.† The materials prepared were characterized by FTIR, SEM, TEM, XRD and nitrogen sorption; the details of instruments are shown in the ESI.† The stereoselective adsorption capacity of L-phenylalanine adsorbed by L-Phe-COMS was studied. The stereoselective adsorption of other racemic amino acids (DL-phenylalanine, DL-alanine, DL-lysine and DL-tryptophan) and drugs (naproxen and chlorpheniramine maleate) was also studied. The experimental procedure is represented in detail in the ESI.†
4. Typically, 0.605 g of L-phenylalanine was dissolved in 47.58 mL ultrapure water at constant temperature, and then 2.637 g C18-TMS, 3.337 g TEOS were added stepwise and the mixture was stirred at a constant temperature. The apparent pH value was about 11 until the end of the synthesis. The resulting mixtures were poured into a Teflon kettle and maintained at 80 °C for 24 h. Then, the product was washed with deionized water several times till the pH value decreased to approximately 7.0, washed with ethanol three times, centrifuged at 3000 rpm, and the precipitate was dried overnight at 80 °C. Finally, the COMS material was obtained after calcination at 650 °C for 8 h to remove the organic materials. For comparison purposes, samples were also prepared without L-phenylalanine (blank COMS). The major material source is described in the ESI.† The materials prepared were characterized by FTIR, SEM, TEM, XRD and nitrogen sorption; the details of instruments are shown in the ESI.† The stereoselective adsorption capacity of L-phenylalanine adsorbed by L-Phe-COMS was studied. The stereoselective adsorption of other racemic amino acids (DL-phenylalanine, DL-alanine, DL-lysine and DL-tryptophan) and drugs (naproxen and chlorpheniramine maleate) was also studied. The experimental procedure is represented in detail in the ESI.†
Stereoselective adsorption experiments
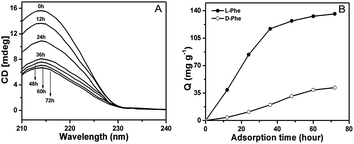
10 mg L-phenylalanine were dissolved by 10 mL ultrapure water in seven round flasks and 40 mg L-Phe-COMS was added to the solutions, respectively. Residual mixtures were collected at different time intervals (0 h, 12 h, 24 h, 36 h, 48 h, 60 h and 72 h) to determine the unabsorbed L-phenylalanine. Control experiments were preformed with blanks containing no L-phenylalanine under the same conditions as for the mixed solution described above. The adsorption kinetics of D-phenylalanine in L-Phe-COMS was further performed to determine the stereoselective adsorption capacity. Afterward, the suspensions were analyzed by circular dichroism spectra (CD) analysis.To further investigate the stereoselective adsorption ability of this material, 10 mg D,L-phenylalanine, D,L-alanine and D,L-lysine were dissolved in 10 mL ultrapure water, DL-tryptophan, naproxen and chlorpheniramine maleate were dissolved in 10 mL methanol, respectively; and then 40 mg L-Phe-COMS was added to the above solutions. Finally, the residual mixture was collected after 72 h to determine the unabsorbed molecules by CD analysis.
Results and discussion
Characterization of the prepared L-Phe-COMS
FTIR was performed to validate the removal of organic materials. Peaks at 2921.43 cm−1 and 2848.92 cm−1 represent the bending vibrations of –C–H in C18-TMS belonging to organic materials and the absence of these two peaks in L-Phe-COMS indicates the complete removal of organic materials (Fig. S1A†). The spectrum of L-Phe-COMS shows the successful formation of a silica skeleton due to the emerging stretching vibration (1124.43 cm−1) and the bending vibration (850.78 cm−1) of –Si–O–Si–. The XRD patterns of COMS prepared with L-forms of phenylalanine (Fig. 1A) show four characteristic peaks (at 2.1°, 3.5°, 4.0°, and 5.3°) of the MCM-41 hexagonal structure, which could be indexed as (100), (110), (200), and (210).18 In addition, the material retained its XRD order upon calcination. However, an evident contraction was obtained from 2.15 to 1.75 nm (d-spacing values obtained from Bragg's law). The XRD pattern of a blank material prepared without phenylalanine is included for comparison, revealing that this material did not possess the MCM-41-type structure.N2 adsorption–desorption isotherms and corresponding pore size distribution of extracted mesoporous materials are shown in Fig. 1. The BJH pore diameters were in the narrow range from 2.3 nm to 2.5 nm, and the values of the BET surface area (m2 g−1) and pore volume (cm3 g−1) were 730 and 0.47, respectively, obtained by N2 relative pressure arranging from 0.05 to 0.20. N2 adsorption–desorption isotherms are assigned to a type-IV, with the absence of a hysteresis loop consistent with pore diameters below approximately 4 nm, characteristic for MCM-41 materials,19 which suggested the mesoporous structure of L-Phe-COMS. The SEM image of the as-obtained L-Phe-COMS showed irregular nanoparticles (Fig. 2A). The TEM image of the template-unremoved COMS (Fig. 2B) clearly exhibited the existence of ordered mesopores and the ordered mesoporous feature still existed in the calcined L-Phe-COMS (Fig. 2C and D). Hexagon channels are distinctly observed in Fig. 2D, different from Fig. 2B. Furthermore, insets of Fig. 2C and D are fast Fourier transform (FFT) diffractograms along perpendicular (C) and parallel (D) directions to the channel axis, which are assigned to two orientations of the p6mm plane of feature characteristics MCM-41 materials.
Effect of L-phenylalanine, C18-TMS, TEOS and H2O on the preparation of L-Phe-COMS
For the optimum preparation of L-Phe-COMS, COMS-n (n = 1–9) was synthesized by different molar rations of precursors as shown in Table 1, with the same procedure described above. To investigate the effect on mesoporous pore order, in which the COMS-2 was marked as L-Phe-COMS in this work. It was observed that the sodium hydroxide concentration significantly affected the mesoporous order. The XRD of COMS-1 in Fig. S2† showed lower mesoporous order compared to COMS-2, which might result from the faster TEOS polymerization and slower arrangement of the ordered pore structure into the silica framework in a weak alkaline environment. Appropriate amounts of OH−, which compressed the double electric layer and weakened the interaction among the charged surfactants, arranged the surfactants more closely and promoted surfactant molecule aggregation into micelles. Furthermore, the closely arranged surfactants could promote silica aggregation on the micelles. However, the crippling interaction between organic–inorganic compounds resulted from an overwhelming concentration of OH− in the micelles, decreasing the mesoporous order (Fig. S2,† COMS-3). Silicon polymerization could also be inhibited and an unfavourable monomer form would start to exist due to excessive OH−, under which circumstances it would be difficult to obtain a solid product and a mesoporous structure. Overall, the amounts of OH− played a key role in morphology and mesoporous order. The ratio of C18-TMS/TEOS also had an effect on the mesoporous order. The silica skeleton was difficult to form because of the inadequate TEOS when the ratio was high (Fig. S3,† COMS-4), whereas a higher ratio would affect the formation of micelles and decrease the mesoporous order. It was shown that a higher initial temperature was favourable for the mesoporous order (Fig. S4,† COMS-6). However, the mesoporous order would be reduced at the highest temperature because of the micelle order decreasing (Fig. S4,† COMS-7). An appropriate reaction time played a role in the mesoporous order, which was observed from Fig. S5† (COMS-8, and COMS-9).| Samples | L-Phe | NaOH | C18-TMS | TEOS | H2O | Initial temperature (°C) | Reaction time (h) |
|---|---|---|---|---|---|---|---|
| a Note: COMS-2 was marked as L-Phe-COMS. | |||||||
| COMS-1 | 2 | 2 | 1 | 6 | 1000 | 25 | 24 |
| COMS-2a | 2 | 4 | 1 | 6 | 1000 | 25 | 24 |
| COMS-3 | 2 | 8 | 1 | 6 | 1000 | 25 | 24 |
| COMS-4 | 2 | 4 | 1 | 3 | 1000 | 25 | 24 |
| COMS-5 | 2 | 4 | 1 | 9 | 1000 | 25 | 24 |
| COMS-6 | 2 | 4 | 1 | 6 | 1000 | 0 | 24 |
| COMS-7 | 2 | 4 | 1 | 6 | 1000 | 50 | 24 |
| COMS-8 | 2 | 4 | 1 | 6 | 1000 | 25 | 12 |
| COMS-9 | 2 | 4 | 1 | 6 | 1000 | 25 | 36 |
Under alkaline conditions, the electrostatic interaction between solvated silicate anions and cationic surfactant assemblies (often referred to as the organic template), combined with hydrophobic interactions of the nonpolar surfactant tails, drives the formation of mesostructured silica.16 It was deduced that the hydrolyzed C18-TMS surfactant molecule condensed to form positively charged dimers. In addition, both negatively charged amino acid molecules and silica species formed an electrostatic interaction with the dimers under basic conditions, and the dimers further organized into micelles because of the hydrophobic interaction of the nonpolar surfactant tails (C18-TMS). Moreover, the silica species combined with the C18-TMS by covalent bonds and formed the silica skeleton. Therefore, mesostructured silica was formed and the chirality was transferred from the micelles into the ordered mesoporous silica.
In summary, C18-TMS participated in both the formation of the silica skeleton (as silica source) and the chiral pore channel (as a type of positively charged surfactant). In addition, amino acids exhibited electrostatic interactions with the positively charged surfactant and also participated in the formation of the chiral pore channel.
Stereoselective adsorption of L-phenylalanine on L-Phe-COMS
In this work, we take the advantages of unique structural features of L-Phe-COMS and imprinting chirality as a robust adsorbent for stereoselective adsorption capacity. First, the stereoselective adsorption capacity of L-Phe-COMS for L-phenylalanine was investigated. The CD spectra of the DL-Phe solution adsorbed by L-Phe-COMS and blank COMS (obtained without L-Phe), respectively, indicated the L-Phe molecule resulted in the stereoselective adsorption. Furthermore, it was observed that the concentration of L-phenylalanine in the solution decreased time-dependently (Fig. 3A). Over half of the L-phenylalanine (1.0 mg mL−1) was adsorbed on 40 mg of L-Phe-COMS after 72 h. The concentration of L-phenylalanine in the solution can be obtained from the eqn (1):| θ = 100[θ]lc/M | (1) |
The stereoselective adsorption factor between L-phenylalanine and D-phenylalanine in solution, having interacted with L-Phe-COMS for 72 h can be calculated from the eqn (2):
| α = QL-Phe/QD-Phe | (2) |
After the chiral recognition of L-Phe-COMS was identified, this chiral imprinting ordered mesoporous solid silicate was further explored to separate other racemic mixtures. Fig. 4 shows a stronger chiral recognition ability of L-Phe-COMS for amino acids (D,L-phenylalanine, D,L-alanine, D,L-tryptophan, and D,L-lysine) compared to some racemic drugs (chlorpheniramine maleate and naproxen). Chlorpheniramine maleate and naproxen were hardly absorbed on calcined L-Phe-COMS, as a result of both no amino acid groups and larger molecular size. In comparison to alanine, a long alkyl chain hampers the effective adsorption of lysine on L-Phe-COMS. Thus, the L-form of the guests, simultaneous with suitable molecular size and the presence of a benzene group, was appropriate for the stereoselective adsorptive separation of racemic guests. The results observed above showed that the high adsorption capacity and stereoselective separation of racemic guests was favoured by the chirality imprinting microenvironment.
Preliminary mechanism of stereoselective adsorption of L-Phe-COMS
The stereoselective adsorption mechanism of the L-Phe-COMS could be illustrated in view of COMS formation process. Differential stereoselective adsorption may result from the molecule chirality imprinted in the pore channel as shown in Scheme 1. In this experiment, the chirality transferred from the L-phenylalanine to the silica skeleton enabled more stereoselective adsorption of L-phenylalanine compared to D-phenylalanine. The supramolecule formed in the synthesis also had an effect on the stereoselective adsorption. During the synthesis of L-Phe-COMS, C18-TMS and its dimers or trimmers presented an electrostatic interaction with L-phenylalanine and formed micelles. Moreover, the head group of C18-TMS was dragged and twisted by L-phenylalanine; thus, forming the chiral pore channel. Consequently, the stereoselective recognition ability of L-Phe-COMS may result from not only the chirality imprinted in the pore channel, but also from the chiral pore channel formed by the twisted micelle, which was constituted by the L-phenylalanine and C18-TMS.17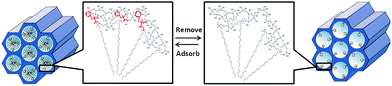 | ||
| Scheme 1 A representative scheme for L-Phe-COMS with an ordered MCM-41-type structure before and after the removal of the chiral introducer and adsorption of the target molecule. | ||
Conclusion
In summary, L-phenylalanine imprinting chiral ordered mesoporous silica (L-Phe-COMS) was straightforward synthesized by combining tetraethyl orthosilicate and quaternized aminosilane silica sources together with L-phenylalanine as a chiral imprinted reagent. The obtained L-Phe-COMS showed several features, such as highly ordered mesoporous structure, narrow pore size distribution, ranging from 2.3 nm to 2.5 nm, large specific surface area of 730 cm2 g−1 and high pore volume of 0.47 cm3 g−1. C18-TMS participated in the formation of the chiral pore channel (as a type of positively charged surfactant), as well as the silica skeleton (as silica source). Amino acids participate in the formation of the chiral pore channel through a similar electrostatic interaction with the positively charged surfactant. An exceptional stereoselective adsorption capacity of up to 3.24 for L-phenylalanine over D-phenylalanine was obtained using L-Phe-COMS in an aqueous solution. Unique structural features and the stereoselective adsorption capacity of COMS make it an alternative candidate for a multitude of applications involving asymmetric catalysis and enantiomeric separation.Acknowledgements
The authors gratefully acknowledge the financial support for this work from the Natural Science Foundation of Xinjiang Uygur Autonomous Region (Grant nos 201233146-7), the Natural Science Foundation of Jiangsu Province (Grant nos BK20130654).References
- S. MacQuarrie, M. P. Thompson, A. Blanc, N. J. Mosey, R. P. Lemieux and C. M. Crudden, J. Am. Chem. Soc., 2008, 130, 14099 CrossRef CAS PubMed.
- S. Polarz and A. Kuschel, Adv. Mater., 2006, 18, 1206 CrossRef CAS.
- G. Zhu, D. Jiang, Q. Yang, J. Yang and C. Li, J. Chromatogr. A, 2007, 1149, 219 CrossRef CAS PubMed.
- G. Zhu, H. Zhong, Q. Yang and C. Li, Microporous Mesoporous Mater., 2008, 116, 36 CrossRef CAS PubMed.
- A. Gabashvili, D. D. Medina, A. Gedanken and Y. Mastai, J. Phys. Chem. B, 2007, 111, 11105 CrossRef CAS PubMed.
- X. Wu, S. Ji, Y. Li, B. Li, X. Zhu, K. Hanabusa and Y. Yang, J. Am. Chem. Soc., 2009, 131, 5986 CrossRef CAS PubMed.
- X. Liu, W. Zhuang, B. Li, L. Wu, S. Wang, Y. Li and Y. Yang, Chem. Commun., 2011, 47, 7215 RSC.
- B. Di, L. Cheng, Q. Jiang, M. Su and W. Hao, New J. Chem., 2013, 37, 1603 RSC.
- T. Yokoi, Y. Sakamoto, O. Terasaki, Y. Kubota, T. Okubo and T. Tatsumi, J. Am. Chem. Soc., 2006, 128, 13664 CrossRef CAS PubMed.
- R. Bhushan and S. Dixit, Biomed. Chromatogr., 2012, 26, 962 CAS.
- S. Lacasta, V. Sebastian, C. Casado, A. Mayoral, P. Romero, A. Larrea, E. Vispe, P. Lopez-Ram-de-Viu, S. Uriel and J. Coronas, Chem. Mater., 2011, 23, 1280 CrossRef CAS.
- C. Casado, J. Castán, I. Gracia, M. Yus, A. Mayoral, V. Sebastián, P. López-Ram-de-Viu, S. Uriel and J. Coronas, Langmuir, 2012, 28, 6638 CrossRef CAS PubMed.
- R. Bhushan and S. Tanwar, J. Chromatogr. A, 2010, 1217, 1395 CrossRef CAS PubMed.
- Z. F. Zhang, G. L. Yang, D. X. Wang, G. J. Liang and Y. Chen, J. Liq. Chromatogr. Relat. Technol., 2004, 27, 813 CrossRef CAS PubMed.
- J. D. Senra, L. F. B. Malta, P. M. N. Ceva-Antunes, R. J. Correa and O. A. C. Antunes, J. Braz. Chem. Soc., 2007, 18, 1367 CrossRef CAS PubMed.
- J. Fan, S. W. Boettcher, C.-K. Tsung, Q. Shi, M. Schierhorn and G. D. Stucky, Chem. Mater., 2008, 20, 909 CrossRef CAS.
- Z. Guo, Y. Du, Y. Chen, S.-C. Ng and Y. Yang, J. Phys. Chem. C, 2010, 114, 14353 CAS.
- C. Kresge, M. Leonowicz, W. Roth, J. Vartuli and J. Beck, Nature, 1992, 359(6397), 710 CrossRef CAS.
- Q. Huo, D. I. Margolese and G. D. Stucky, Chem. Mater., 1996, 8(5), 1147 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra11302k |
| This journal is © The Royal Society of Chemistry 2014 |