Magnetic, X-ray and Mössbauer studies on magnetite/maghemite core–shell nanostructures fabricated through an aqueous route†
Abstract
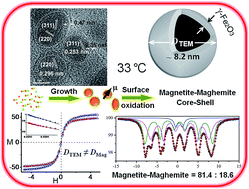
Uniform 6–13 nm sized 0D superparamagnetic Fe3O4 nanocrystals were synthesized by an aqueous ‘co-precipitation method’ under a N2 atmosphere as a function of temperature to understand the growth kinetics. The crystal phases, surface charge, size, morphology and magnetic characteristics of as-synthesized nanocrystals were characterized by XRD, Raman spectroscopy, FTIR, TG-DTA, BET surface area, dynamic light scattering along with zeta potential, HR-TEM, EDAX, vibrating sample magnetometry and Mössbauer spectroscopy. TEM investigation revealed highly crystalline spherical magnetite particles in the 8.2–12.5 nm size range. The kinetically controlled as-grown nanoparticles were found to possess a preferential (311) orientation of the cubic phase, with a highest magnetic susceptibility of ∼57 emu g−1. The Williamson–Hall technique was employed to evaluate the mean crystallite size and microstrain involved in the as-synthesized nanocrystals from the X-ray peak broadening. In addition to FTIR and Raman spectra, Rietveld structural refinement of XRD confirms the magnetite phase with 5–20% maghemite in the sample. VSM and Mössbauer spectral data allowed us to fit the magnetite/maghemite content to a core–shell model where the shell is 0.2–0.3 nm thick maghemite over a magnetite core. The activation energy of <10 kJ mol−1 calculated from an Arrhenius plot for the complex process of nucleation and growth by diffusion during synthesis shows the significance of the precipitation temperature in the size controlled fabrication processes of nanocrystals. Brunauer–Emmett–Teller (BET) results reveal a mesoporous structure and a large surface area of 124 m2 g−1. Magnetic measurement shows that the particles are ferromagnetic at room temperature with zero remanence and zero coercivity. This method produced highly crystalline and dispersed 0D magnetite nanocrystals suitable for biological applications in imaging and drug delivery.

 Please wait while we load your content...
Please wait while we load your content...