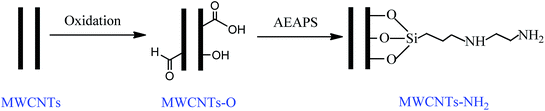
Pretreated multiwalled carbon nanotube adsorbents with amine-grafting for removal of carbon dioxide in confined spaces
Bin Yanga,
Huirong Hua,
Qingni Yuab,
Xingwang Zhang*a,
Zhongjian Lia and
Lecheng Leia
aKey Laboratory of Biomass Chemical Engineering of Ministry of Education, Department of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310027, China. E-mail: xwzhang@zju.edu.cn; Fax: +86-571-87952525; Tel: +86-571-87952525
bChina Astronaut Research and Training Center, Beijing 100094, China
First published on 20th October 2014
Abstract
Three different methods, including thermal treatment, treatment with HNO3 and O2 oxidation, were used to pretreat multiwalled carbon nanotubes (MWCNTs) before grafting with N-(2-aminoethyl)-3-aminopropyltrimethoxysilane (AEAPS). The types and contents of the O-containing groups generated by the various pretreatment methods were quantified and the corresponding amine-grafting reactions investigated. The alkoxyl groups of AEAPS react with the O-containing groups on the pretreated MWCNTs through silylation reactions in which there is no carboxyl acids induced by O2 gas oxidation. The grafted primary amino groups can be accessible to capture the maximum amount of CO2. A dynamic fixed-bed system was used to characterize the adsorption behavior of low concentrations of CO2. The adsorption/desorption operations of 10 repeated cycles were investigated to verify the sustained excellent performance. The highest CO2 adsorption capacity of 0.64 mmol g−1 was achieved by the O2-oxidized MWCNTs with AEAPS-grafting, which is almost 7.1 times the CO2 adsorption capacity of the oxidized MWCNTs without amine-grafting. This indicates that O2 gas oxidation is simple to operate and highly efficient for the pretreatment of MWCNTs. The adsorbent obtained has a high capacity, high thermal stability, high tolerance to moisture and low regeneration cost and shows promise for the direct capture of low concentrations of CO2 in confined spaces.
1. Introduction
Carbon capture and sequestration technologies have received increasing attention in recent years1,2 as a means of reducing the impacts of the increasing concentration of CO2 in the atmosphere on the global climate. It is estimated that approximately one-third of global carbon emissions are emitted from distributed sources, such as transportation vehicles, where low concentrations of CO2 (about 400 ppm) need to be directly captured from the ambient air (“air capture”).3 Capture technologies for the high concentrations of CO2 generated from large point sources, such as coal-fired power plants (about 10% CO2), are relatively mature.4,5 In addition, CO2 capture is also required in confined spaces, such as submarines, space shuttles and space stations, where CO2 is emitted by humans during breathing, from mechanical equipment and during the oxidation of various materials.6,7 High concentrations of CO2 in such enclosed spaces can threaten the survival of humans.8,9The various approaches used to capture CO2 from a mixture of gases include absorption, cryogenic distillation, adsorption and membrane separation.10–13 Chemical absorption processes using lithium hydroxide or liquid amines are mature commercial technologies and have a high capacity for CO2 capture. However, there are drawbacks to these techniques, such as high operational costs, container corrosion, their high energy intensity, and viscosity and foaming issues.14 Cryogenic distillation and membrane separation processes generally have low efficiencies and high costs, especially for capture from ambient air.10 Adsorption using porous solid sorbents, including carbon materials,15,16 polymers,6 silica materials17–20 and metal–organic frameworks,21 is more promising because these materials require less energy for regeneration.22
In addition to physical adsorbents, amine-functionalized adsorbents are attractive because they have active alkaline sites that can be used for the adsorption of CO2.15,23 These chemical adsorbents are typically prepared by physically immobilizing liquid amines or chemically grafting amino groups onto the surface of a porous support.24 A higher loading of amine can be achieved by an impregnation method; however, these adsorbents exhibit low thermal stability and poor distribution of the amine.25–28 The CO2 adsorption capacity decreases gradually with increasing numbers of adsorption/desorption cycles as a result of the leaching of amines and the efficiency of these adsorbents is low.29 Intensive research efforts have been devoted to chemically grafting amines onto silica-based solid supports such as SBA-16,17 SBA-15,30,31 and MCM-41.29,32 Carbon nanotubes (CNTs), however, are more suitable as supports because of their tolerance to moisture compared with silica-based adsorbents.22,33–35 It has been shown that CNT-based adsorbents are light in weight, have high surface areas and high thermal and chemical stabilities. In addition, their chemical reactivity can be controlled.36–38 Studies of their thermodynamics and regeneration have shown that amine-grafted CNTs are potential cost-effective sorbents for CO2 capture.33,34
The introduction of O-containing groups onto supports by chemical pretreatment is an important method of chemical grafting that can be used to covalently link amine functional groups. The most commonly used pretreatment methods are acid oxidation, UV photo-oxidation, thermal treatment, and plasma and gas phase oxidation.37,39,40 Velasco-Santos et al.40 used an arc-discharge method to oxidize carbon nanotube surfaces to improve the chemical bonding with different polymer materials. They showed that the trisilanol groups on organosilanes can react with the hydroxyl groups on the pre-oxidized nanotube surface and therefore the organosilanes will be attached to the surface of the nanotubes.40 However, the arc-discharge treatment needs to be conducted at high voltages and the high costs of the power supply have restricted its use in industrial applications. Acid treatment gives relatively high yields of functional groups, including carboxylic acid, phenol, carbonyl, carboxylic anhydride and lactone groups.34 However, acid treatment is difficult and time-consuming with respect to the required rinsing and drying operations. Gas phase oxidation is more convenient and effective, particularly as it is contaminant-free, and O-containing groups including phenol, carbonyl/quinone and lactone groups can be generated.37,41 Thermal treatment can selectively remove some functional groups, such as carboxylic acid. An intensive study of the correlation between O-containing groups on the oxidized CNTs and the efficiency of the silylation reaction was conducted by Gaspar et al.37 The highest efficiency was achieved with oxidation by HNO3 with subsequent thermal treatment; this method gave a high content of O-containing groups, including phenol groups, but without carboxylic acids. However, these workers did not concentrate on the study of CO2 capture.
Some pretreatment methods, including thermal, acid and NaOH treatments, have been used only to purify CNTs before amine-grafting22,35,42 and the tuning of the O-containing functional groups induced by the oxidative methods on amine-grafting for CO2 capture has seldom been investigated. In the work reported here, multiwalled CNTs (MWCNTs) were first pretreated by acid oxidation, thermal treatment or gas phase oxidation and then N-(2-aminoethyl)-3-aminopropyltrimethoxysilane (AEAPS) was used to tether the pretreated MWCNTs. We investigated the effects of the type and content of the O-containing groups on the corresponding amine-grafting reaction. The amine-grafting efficiencies for CO2 capture and the mechanisms for the adsorption of CO2 were also studied. The textural properties, surface chemical composition and morphologies of the resulting modified MWCNTs were characterized by N2 adsorption isotherms, thermogravimetry, X-ray photoelectron spectrometry, Fourier transform infrared spectrometry, elemental analysis and scanning electron microscopy. The amine-grafted MWCNTs obtained were then used for removal of CO2 from confined spaces, where the representative CO2 level is about 2 vol%.8 The effects of the operating temperature and humidity of the gas mixture on the adsorption behavior of CO2 and the repeatability of the procedure were studied.
2. Experimental section
2.1 Preparation of amine-grafted MWCNTs
Thermal treatment. The pristine MWCNTs were thermally treated in a fused-silica tube with N2 gas (100 mL min−1) at 300 °C for 1 h and then cooled to room temperature.
Acid oxidation. About 4 g of pristine MWCNTs were refluxed in 300 mL of 68 wt% HNO3 at 80 °C for 8 h and then cooled to room temperature. The samples were then filtered and washed with deionized water until the pH of the filtrate became neutral. The samples obtained were then dried overnight in a vacuum at 60 °C. When the raw MWCNTs were treated by mixture of HNO3 and H2SO4 (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) at 80 °C for 4 h, the obtained samples were denoted as m-MWCNTs.
3 v/v) at 80 °C for 4 h, the obtained samples were denoted as m-MWCNTs.
Gas oxidation. The pristine MWCNTs were heated to 500 °C at 4 °C min−1 in a 5 vol% O2/N2 gas mixture (100 mL min−1) and kept at 500 °C for 3 h. The samples were then cooled to room temperature under an N2 atmosphere.
2.2 Characterization of adsorbents
The materials were characterized texturally by N2 physical adsorption/desorption isotherms (TriStar 3000, Micrometrics) at −196 °C. The samples were vacuum-dried at 120 °C for 3 h before determination. The Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods were used to calculate the surface area and average pore diameter, respectively, from the adsorption branch of the isotherms. The total pore volume was calculated from the amount of N2 adsorbed at P/P0 = 0.98 and the micro surface areas and volume were calculated by the t-plot method. The surface morphological features were observed by field-emission scanning electron microscopy (SU-70 microscope, Hitachi) at 3 kV. The thermal stabilities were obtained by thermogravimetry and differential scanning calorimetry (SDT Q600, TA Instruments) in an N2 atmosphere at a flow-rate of 120 mL min−1. The functional groups present below the corresponding decomposition temperature were determined quantitatively. The sample was heated from room temperature to 900 °C at a heating rate of 10 °C min−1 in an N2 atmosphere. X-ray photoelectron spectrometry (Amicus spectrometer, Shimadzu) using a monochromatized Al Kα radiation source (1486.7 eV) and Fourier transform infrared spectrometry (Nicolet 6700 instrument) were used to study and identify the surface chemical properties, particularly the determination of the amino groups. The elemental content of the adsorbents was quantitatively determined using a Flash EA 1112 instrument (Thermo Finnigan).2.3 Adsorption experiments
The experimental studies of the adsorption of CO2 were conducted in a dynamic fixed-bed system (Fig. 1). The glass adsorption column packed with 1 g of adsorbent was placed in a temperature-controlled box (AT-950, Science Instrument, China) to maintain a constant temperature. The internal diameter and total length of the column were approximately 1 and 20 cm, respectively. A mixture of pure N2 and CO2 was blended in a glass bottle and the influent CO2 concentration was kept at 2 vol%. Mass flow controllers (D07-19C, Sevenstar Electronics, China) were used to control the flow-rate of the influent mixture gas at 50 mL min−1. The influent gas mixture was passed through the adsorption column and CO2 was adsorbed and removed. Once the CO2 adsorption process had reached equilibrium, the saturated adsorbents were then desorbed at an adsorption column temperature of 75 °C and 5 kPa vacuum. After 5 min, the concentration of desorbed CO2 in the effluent gas decreased to zero, indicating that the adsorption ability of the adsorbents had been recovered and that they were available for the next round of the adsorption study.Gas chromatography using a chromatographic column (3.0 mm × 4.0 mm, Proapak QS) and a thermal conductivity detector (GC9790, Fuli, China) was used to automatically detect the CO2 concentration of the influent and effluent gas streams. The operating temperatures of the injector, column and detector were set at 100 °C, 140 °C, and 150 °C, respectively. The moisture content of the influent gas mixture was generated from a water bubbler in which the stream of N2 was bubbled through deionized water and the amount of water carried was controlled by adjusting the temperature of the water-bath. A higher temperature resulted in a higher water vapor content. The water content was calculated by the following equation.
 | (1) |
The CO2 adsorption capacity (q, mmol g−1) at a certain time (t, min) was calculated as follows:
 | (2) |
The calculation of the amine-grafting efficiencies for CO2 capture (η, CO2/N molar ratio) recommended by Sculley and Zhou,43 which indicates how many effective amino groups per total weight of adsorbent are accessible to capture CO2, was evaluated:
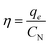 | (3) |
3. Results and discussion
3.1 CO2 adsorption performance of modified MWCNTs
Fig. 2 shows the CO2 equilibrium adsorption capacity of the modified MWCNTs. The pre-oxidative MWCNTs showed a dramatic enhancement in their CO2 equilibrium adsorption capacity after AEAPS-grafting for each pretreatment method. This may be attributed to the presence of sufficient effective amino groups on the surfaces of the AEAPS-grafted MWCNTs to capture a greater amount of CO2. The CO2 adsorption capacity of the o-MWCNTs–AEAPS was 0.64 mmol g−1, which is almost 7.1 times that of the o-MWCNTs (0.09 mmol g−1) before AEAPS-grafting. The CO2 adsorption capacities of the t-MWCNTs–AEAPS and h-MWCNTs–AEAPS were 0.38 mmol g−1 and 0.36 mmol g−1, respectively, which are both significantly improved compared with the MWCNTs without AEAPS-grafting. However, it can be seen that oxidation with O2 produces higher CO2 adsorption capacities after AEAPS-grafting than the thermal and HNO3 pretreatment methods.Various pretreatment methods have been reported previously for the purification of MWCNTs for amine-grafting to improve the adsorption of CO2. For example, Su et al.42 reported amine-modified MWCNTs pretreated by NaOH that had a CO2 adsorption capacity of 0.984 mmol g−1 at 20 °C for 15 vol% CO2. Gui et al.35 used H2SO4/HNO3 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vol/vol) as a pretreatment for the amine-grafting of MWCNTs and the CO2 uptake at 60 °C for 5% CO2 was 1.71 mg g−1. The relatively higher adsorption capacity was mainly attributed to the high level of treated CO2. However, few comparative studies of different pretreatments on AEAPS-grafting for CO2 capture at low concentrations have been reported, in particular the potential advantages of oxidation by O2.
1 vol/vol) as a pretreatment for the amine-grafting of MWCNTs and the CO2 uptake at 60 °C for 5% CO2 was 1.71 mg g−1. The relatively higher adsorption capacity was mainly attributed to the high level of treated CO2. However, few comparative studies of different pretreatments on AEAPS-grafting for CO2 capture at low concentrations have been reported, in particular the potential advantages of oxidation by O2.
3.2 Characterization of the modified MWCNTs
Textural properties. Fig. 4 shows the N2 adsorption/desorption isotherms of the pretreated and AEAPS-modified MWCNTs. The IV shape and H1 type of hysteresis loop according to the IUPAC classification indicate that all the samples exhibit a mesoporous structure with cylindrical pores, in spite of being pretreated and amine-grafted.31 The sharp increment in the adsorption isotherms at high P/P0 values and the adsorption hysteresis loop may be caused by capillary condensation in the mesoporous pores. The o-MWCNTs had the highest N2 adsorption capacity among the pretreated MWCNTs (Fig. 4a), indicating that the highest porosities are obtained after O2 oxidation. However, no significant changes were seen in the N2 adsorption isotherms after the thermal and HNO3 pretreatments. Table 1 gives the physical properties obtained and provides detailed information about the variations between the different pretreatments. The specific surface area (SBET) and pore volume (Vp) of the o-MWCNTs are 151.8 m2 g−1 and 0.394 cm3 g−1, respectively, an increase of almost 18.8% and 15.9% compared with the pristine MWCNTs. The SBET and Vp values of t-MWCNTs and h-MWCNTs show only slight changes, as expected. High specific surface areas and pore volumes are beneficial for amine-grafting and CO2 transport in MWCNTs. Table 1 also shows that amine-grafting leads to a drastic decrease in the surface area compared with the pretreated precursor MWCNTs as a result of the introduction of the organic amines. The SBET value of t-MWCNTs–AEAPS, h-MWCNTs–AEAPS and o-MWCNTs–AEAPS are 102.74 m2 g−1, 66.22 m2 g−1, and 67.05 m2 g−1, respectively, which is a reduction of almost 19.1%, 47.6%, and 55.8%, respectively. The grafted amines are the main contributors to the partial or complete blockage of the pores and result in the decrease in the SBET and Vp values.37 However, the SBET value of the modified MWCNTs did not agree with the CO2 adsorption capacity obtained. The SBET value of t-MWCNTs–AEAPS is relatively higher, indicating that the AEAPS loading may be lower. The average pore diameters all increased after amine-grafting as a result of the coverage of the well-distributed and uniform AEAPS. The average pore diameters of the t-MWCNTs–AEAPS, h-MWCNTs–AEAPS and o-MWCNTs–AEAPS are 13.3 nm, 11.8 nm, and 11.1 nm, respectively, increased by almost 11.3%, 5.9%, and 6.63%, respectively. The thermal treatment resulted in higher average pore diameters because it removed only some of the original oxygen groups from the pristine MWCNTs and the AEAPS were subsequently mainly grafted onto the sidewalls of the nanotubes. HNO3 or O2 oxidation can occur on the end-caps as well as on the sidewalls of the nanotubes, generating more O-containing groups.
 | ||
| Fig. 4 N2 adsorption (solid lines)/desorption (broken lines) isotherms of (a) pretreated and (b) AEAPS-grafted MWCNTs. | ||
| Sample | SBET (m2 g−1) | Vp (cm3 g−1) | Average pore diameter (nm) |
|---|---|---|---|
| MWCNTs | 127.8 | 0.340 | 10.6 |
| t-MWCNTs | 126.9 | 0.379 | 11.9 |
| h-MWCNTs | 126.3 | 0.353 | 11.2 |
| o-MWCNTs | 151.8 | 0.394 | 10.4 |
| t-MWCNTs–AEAPS | 102.7 | 0.341 | 13.3 |
| h-MWCNTs–AEAPS | 66.2 | 0.196 | 11.8 |
| o-CMWNTs–AEAPS | 67.1 | 0.186 | 11.1 |
Morphology. Fig. 5 shows the morphologies of the pretreated MWCNTs and corresponding AEAPS-grafted MWCNTs. There was no obvious change between the t-MWCNTs and pristine MWCNTs (Fig. 5a and its inset, respectively). The surfaces of the h-MWCNTs (Fig. 5b) and o-MWCNTs (Fig. 5c) appear clear because most of the impurities were removed by the HNO3 or O2 treatment. As a result of the aggressive treatment of the O2 oxidation, the open and cutoff structures of the MWCNTs can be seen more readily (highlighted by the circles in Fig. 5c). With respect to the corresponding AEAPS-grafted samples, it is observed that AEAPS was successfully tethered onto the nanotube surfaces. The AEAPS loading on the t-MWCNTs was relatively scattered and low (Fig. 5d). The h-MWCNTs–AEAPS shown in Fig. 5e tends to significant cross-linking. This may be related to the limited diffusion of CO2 to the amino groups deep inside the pores and possibly to blocking of the pore entrances. The CO2 adsorption capacities obtained in these instances were low. The nanotube density of o-MWCNTs–AEAPS was large as a result of the coverage of the well-distributed and uniform AEAPS (Fig. 5f). This shows that O2 oxidation is suitable for AEAPS-grafting and that the uniform distribution of amine groups obtained results in the capture of more CO2.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in MWCNTs and the band at 1200 cm−1 is associated with the scissoring vibration of C–C bonds and the stretching vibration of C–O bonds.45 The broad band at 3440 cm−1 results from the presence of free and associated hydroxyl groups as a result of intercalated water and structural hydroxyl groups (–COOH and –COH). The occurrence of bands at 2920 cm−1 and 2850 cm−1 verifies the presence of C–H bonds from the alkyl and alkoxyl groups in aminosilane. In particular, the band at 1116 cm−1 is assigned to the asymmetrical stretching vibration of the Si–O–R bond, accompanied by the bending vibration of Si–C and Si–OH bonds. The weak band at 1456 cm−1 is due to the bending vibration of the N–H bond in aminosilane.46 This demonstrates that aminosilane has been successfully grafted onto the pretreated MWCNTs by the silylation reactions.
C bonds in MWCNTs and the band at 1200 cm−1 is associated with the scissoring vibration of C–C bonds and the stretching vibration of C–O bonds.45 The broad band at 3440 cm−1 results from the presence of free and associated hydroxyl groups as a result of intercalated water and structural hydroxyl groups (–COOH and –COH). The occurrence of bands at 2920 cm−1 and 2850 cm−1 verifies the presence of C–H bonds from the alkyl and alkoxyl groups in aminosilane. In particular, the band at 1116 cm−1 is assigned to the asymmetrical stretching vibration of the Si–O–R bond, accompanied by the bending vibration of Si–C and Si–OH bonds. The weak band at 1456 cm−1 is due to the bending vibration of the N–H bond in aminosilane.46 This demonstrates that aminosilane has been successfully grafted onto the pretreated MWCNTs by the silylation reactions.
Table 2 presents the quantitative results of the X-ray photoelectron spectrometry analysis of the obtained samples. The highest O content of 7.8% was achieved by HNO3 pretreatment. Elemental Si and N appeared in all the samples, indicating that AEAPS was successfully grafted onto the pretreated MWCNTs. The N contents of the t-MWCNTs–AEAPS, h-MWCNTs–AEAPS, and o-MWCNTs–AEAPS are 6.15%, 8.33% and 7.28%, respectively. However, the high N content is not a linear function with respect to the CO2 adsorption capacity. This is because the CO2 adsorption capacity is dependent on the effective amine groups, especially on the primary amine groups.30 The amine-grafting for o-MWCNTs–AEAPS is more effective because the CO2 adsorption obtained is higher than for the other pretreatment methods.
| Sample | C 1s | O 1s | N 1s | Si |
|---|---|---|---|---|
| MWCNTs | 95.13 | 4.23 | 0.64 | — |
| t-MWCNTs | 97.26 | 2.74 | — | — |
| h-MWCNTs | 91.74 | 7.8 | 0.46 | — |
| o-MWCNTs | 97.26 | 2.74 | — | — |
| t-MWCNTs–AEAPS | 80.59 | 9.43 | 6.15 | 3.84 |
| h-MWCNTs–AEAPS | 72.09 | 14.42 | 8.33 | 5.16 |
| o-MWCNTs–AEAPS | 76.63 | 11.42 | 7.28 | 4.67 |
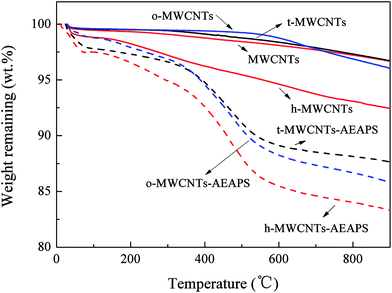
Fig. 7 shows the thermogravimetric curves of the pretreated and AEAPS-grafted MWCNTs. There is a weight loss of less than 2% from room temperature to about 110 °C for all the samples, which is mainly a result of the evaporation of the adsorbed water. The h-MWCNTs show the highest percentage total weight loss among the three pretreated samples, indicating that the amount of O-containing groups introduced by HNO3 treatment is the highest. The weight of the pristine MWCNTs gradually decreases over the whole heating process, verifying that some of the O-containing groups are present on the surface.36,37 The differences in the thermal stabilities based on the decomposition temperature can provide some qualitative information about the O-containing functional groups.37,47 For example, carboxylic acids groups can be selectively removed by thermal treatment at 300 °C. The weight loss of the o-MWCNTs at high temperature is mainly attributed to the decomposition of phenols and carbonyl/quinone groups as well as lactone groups. With respect to the AEAPS-grafted samples, the highest weight loss was obtained by h-MWCNTs–AEAPS, corroborating the result that the AEAPS loading was high in these samples. The high yield of the silylation reaction may be attributed to the large amount of O-containing groups in the pretreated MWCNTs.
The types and contents of the O-containing functional groups on the pretreated MWCNTs can be simply quantified on the basis of the decomposition temperature and the corresponding differential weight loss.37,41 Typical functional groups such as carboxylic acids, phenols and carbonyls/quinones can be related to decomposition temperatures of about 200–450 °C, 600–750 °C and 700–950 °C, respectively.37 The contributions of other groups such as carboxylic anhydrides and lactones were relatively low and can be omitted. We assumed here that the weight loss between 700 °C and 750 °C was equal for phenols and carbonyls/quinones. Table 3 summarizes the types and contents of the O-containing groups in the pretreated MWCNTs. The total contents of O-containing groups in t-MWCNTs, h-MWCNTs, and o-MWCNTs are 2.6%, 5.0% and 3.3%, respectively. For the HNO3 pretreatment, the content of carboxylic acids is 2.4%, a total of 48% of the O-containing groups. However, no carboxylic acids existed in the o-MWCNTs because the O2 pretreatment was conducted at 500 °C; phenols and carbonyl/quinone groups were dominant. This shows that the high N loading obtained can be attributed to the high carboxylic acid content. The absence of carboxylic acids in o-MWCNTs was the basis of the effective AEAPS-grafting, which results in the high CO2 adsorption capacity of the o-MWCNTs–AEAPS.
| Sample | Functional groups | Decomposition temperature (°C) | Δ (%) |
|---|---|---|---|
| t-MWCNTs | Carboxylic acids | 200–450 | 0.6 |
| Phenols | 600–750 | 0.8 | |
| Carbonyl/quinone groups | 700–950 | 1.2 | |
| Total | 2.6 | ||
| h-MWCNTs | Carboxylic acids | 200–450 | 2.4 |
| Phenols | 600–750 | 1.3 | |
| Carbonyl/quinone groups | 700–950 | 1.3 | |
| Total | 5.0 | ||
| o-MWCNTs | Phenols | 600–750 | 1.5 |
| Carbonyl/quinone groups | 700–950 | 1.8 | |
| Total | 3.3 |
3.3 Amine-grafting reactions in MWCNTs used for CO2 capture
The primary amino groups react with CO2 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio and show a higher capacity for CO2 adsorption.48 The low CO2 adsorption capacities obtained for the t-MWCNTs–AEAPS and h-MWCNTs–AEAPS are mainly attributed to the consumption of the primary amino groups by possible side-reactions, including the NH2–silicon polymerization and amide reactions of amines as (Fig. 9). AEAPS is a bifunctional organosilane and the N of the –NH2 group is nucleophilic. The oxygen of the carboxylic acids generated by acid oxidation shows strong electron-withdrawing properties and the carbon connected to the oxygen therefore has a small δ+ value and promotes the nucleophilic attack of the NH2 groups (Fig. 9a). The toluene solvent is a good water-carrying agent and may also promote the amide reaction. Fig. 9b shows the direct attack of the nucleophilic amino groups on the silicon center in the presence of acidic carboxylic acids, where the methoxyl group acts as a leaving group under acid-catalyzed conditions. Polymerization caused by excessive AEAPS-grafting on the MWCNTs surface would block the pores and limit the diffusion of CO2 into the internal pores.34 Although the highest loading of amino groups anchored to the pretreated MWCNTs was obtained by HNO3 pretreatment, some NH2 groups were hindered and were not free to act as active adsorption sites for CO2. Thus the adsorption capacity obtained was low.
1 molar ratio and show a higher capacity for CO2 adsorption.48 The low CO2 adsorption capacities obtained for the t-MWCNTs–AEAPS and h-MWCNTs–AEAPS are mainly attributed to the consumption of the primary amino groups by possible side-reactions, including the NH2–silicon polymerization and amide reactions of amines as (Fig. 9). AEAPS is a bifunctional organosilane and the N of the –NH2 group is nucleophilic. The oxygen of the carboxylic acids generated by acid oxidation shows strong electron-withdrawing properties and the carbon connected to the oxygen therefore has a small δ+ value and promotes the nucleophilic attack of the NH2 groups (Fig. 9a). The toluene solvent is a good water-carrying agent and may also promote the amide reaction. Fig. 9b shows the direct attack of the nucleophilic amino groups on the silicon center in the presence of acidic carboxylic acids, where the methoxyl group acts as a leaving group under acid-catalyzed conditions. Polymerization caused by excessive AEAPS-grafting on the MWCNTs surface would block the pores and limit the diffusion of CO2 into the internal pores.34 Although the highest loading of amino groups anchored to the pretreated MWCNTs was obtained by HNO3 pretreatment, some NH2 groups were hindered and were not free to act as active adsorption sites for CO2. Thus the adsorption capacity obtained was low.
| Support material | Amine type | Method | CN (mmol g−1) | Adsorption capacity | Operating conditions | CO2/N (mol mol−1) | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| mg g−1 | mmol g−1 | Cin (%) | T (°C) | ||||||
| a Cin = influent concentration of CO2; tri = aminoethylaminoethylyaminopropyltrimethoxysilane; PEI = polyethyleneimine; * calculated values. | |||||||||
| t-MWCNTs | AEAPS | Grafting | 1.81 | — | 0.38 | 2 | 25 | 0.21 | This work |
| h-MWCNTs | 2.77 | — | 0.36 | 2 | 0.13 | ||||
| o-MWCNTs | 2.66 | — | 0.64 | 2 | 0.24 | ||||
| o-MWCNTs | 2.67 | — | 1.31 | 15 | 0.49 | ||||
| MCM-41 | Tri | Anhydrous grafting | 5.75* | — | 1.04 | 5 | 25 | 0.181 | Harlick et al.3 |
| PE–MCM-41 | Anhydrous grafting | 6.10* | — | 1.55 | 0.254 | ||||
| PE–MCM-41 | Water-aided grafting | 7.98* | — | 2.65 | 0.332 | ||||
| HMS-C | 45% PEI | Impregnation | 10.19* | 94 | 2.14* | 100 | 75 | 0.21 | Chen et al.49 |
| 70% PEI | 16.33* | 108 | 2.45* | 0.15 | |||||
| HMS-T90 | 60% PEI | 13.93* | 184 | 4.18* | 0.3 | ||||
| 70% PEI | 16.30* | 165 | 3.75* | 0.23 | |||||
| SBA-15 | 30% TEPA + 20% DEA | Impregnation | — | — | — | 100 | 75 | 0.4 | Yue et al.50 |
3.4 Optimization of CO2 capture on AEAPS-grafted MWCNTs
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with the assistance of H2O.32 In contrast, two molecular amino groups were required to bond one molecule of CO2 under anhydrous conditions. Therefore the promoted amine efficiency resulted in a high capacity for CO2 adsorption.
1 with the assistance of H2O.32 In contrast, two molecular amino groups were required to bond one molecule of CO2 under anhydrous conditions. Therefore the promoted amine efficiency resulted in a high capacity for CO2 adsorption.
4. Conclusion
The effects of the type and content of O-containing groups induced on MWCNTs by different pretreatments were investigated. The alkoxyl groups of AEAPS react with the O-containing groups on the pretreated MWCNTs through a silylation reaction. The obtained adsorbents were used to remove CO2 in confined spaces at ambient temperature. A high silylation efficiency can result in high amine loading as a result of the high content of O-containing groups on the pretreated MWCNTs; however, this was not proportional to the high CO2 adsorption capacity. The presence of carboxylic acids generated by HNO3 treatment resulted in side-reactions, including the amide reaction and NH2–silicon polymerization, which consumed the primary amine groups and resulted in a lower capacity. The MWCNTs adsorbents pretreated with O2 and AEAPS-grafting showed a high CO2 adsorption capacity of 0.64 mmol g−1, which is almost 7.1 times that of o-MWCNTs. The amine-grafting efficiencies at 2 vol% and 15 vol% are 0.24 mol CO2/mol N and 0.49 mol CO2/mol N, respectively; the latter is close to the theoretical maximum value of 0.5. The capacity maintained this high level at operating temperatures of up to 40 °C. The adsorbent can be readily recovered and no significant reduction in adsorption was observed after 10 repeated adsorption/desorption cycles. If a lower desorption temperature was applied and one-third of the vacuum value, then this would significantly reduce the energy cost. This indicates that O2 gas oxidation is both simple in operation and highly efficient for the pretreatment of MWCNTs if subsequent grafting with aminosilane is used. The adsorbent obtained has a high capacity for CO2 adsorption, a high thermal stability, a high tolerance to moisture and a low regeneration cost, and is promising for the direct capture of CO2 in confined spaces.Acknowledgements
This work was partially supported by the National Natural Science Foundation of China (NSFC) (nos 21076188 and 21406197), the Natural Science Foundation of Zhejiang Province (LY12E06001) and the Postdoctoral Research Foundation of Zhejiang Province (BSH1302042).References
- Y. Kuwahara, D.-Y. Kang, J. R. Copeland, N. A. Brunelli, S. A. Didas, P. Bollini, C. Sievers, T. Kamegawa, H. Yamashita and C. W. Jones, Dramatic Enhancement of CO2 Uptake by Poly(ethyleneimine) Using Zirconosilicate Supports, J. Am. Chem. Soc., 2012, 134(26), 10757–10760 CrossRef CAS PubMed.
- S. Choi, J. H. Drese and C. W. Jones, Adsorbent Materials for Carbon Dioxide Capture from Large Anthropogenic Point Sources, ChemSusChem, 2009, 2(9), 796–854 CrossRef CAS PubMed.
- P. J. E. Harlick and A. Sayari, Applications of Pore-Expanded Mesoporous Silica. 5. Triamine Grafted Material with Exceptional CO2 Dynamic and Equilibrium Adsorption Performance, Ind. Eng. Chem. Res., 2006, 46(2), 446–458 CrossRef.
- C. M. White, B. R. Strazisar, E. J. Granite, J. S. Hoffman and H. W. Pennline, Separation and Capture of CO2 from Large Stationary Sources and Sequestration in Geological Formations – Coalbeds and Deep Saline Aquifers, J. Air Waste Manage. Assoc., 2003, 53(6), 645–715 CAS.
- A. Samanta, A. Zhao, G. K. H. Shimizu, P. Sarkar and R. Gupta, Post-Combustion CO2 Capture Using Solid Sorbents: A Review, Ind. Eng. Chem. Res., 2011, 51(4), 1438–1463 CrossRef.
- S. Satyapal, T. Filburn, J. Trela and J. Strange, Performance and Properties of a Solid Amine Sorbent for Carbon Dioxide Removal in Space Life Support Applications, Energy Fuels, 2001, 15(2), 250–255 CrossRef CAS.
- R. Serna-Guerrero and A. Sayari, Modeling adsorption of CO2 on amine-functionalized mesoporous silica. 2: kinetics and breakthrough curves, Chem. Eng. J., 2010, 161(1–2), 182–190 CrossRef CAS PubMed.
- P. Moloney, C. Huffman, O. Gorelik, P. Nikolaev, S. Arepalli, R. Allada, M. Springer and L. Yowell, Advanced life support for space exploration: air revitalization using amine coated single wall carbon nanotubes, Mater. Res. Soc. Symp. Proc., 2005, 2005, 59–64 Search PubMed.
- R. Allada, P. Moloney, M. Anderson, F. Smith, S. Arepalli, L. Yowell, J. Chattopadhyay, K. Shah, W. E. Billups and T. Filburn, Nanoscale materials for human spaceflight applications: regenerable carbon dioxide removal using single-wall carbon nanotubes, in SAE Technical Papers, Norfolk, VA, United States, 2006 Search PubMed.
- S. Choi, J. H. Drese, P. M. Eisenberger and C. W. Jones, Application of Amine-Tethered Solid Sorbents for Direct CO2 Capture from the Ambient Air, Environ. Sci. Technol., 2011, 45(6), 2420–2427 CrossRef CAS PubMed.
- Z. Chen, N. Ren, A. Wang, Z. Zhang and Y. Shi, A novel application of TPAD-MBR system to the pilot treatment of chemical synthesis-based pharmaceutical wastewater, Water Res., 2008, 42(13), 3385–3392 CrossRef CAS PubMed.
- W. Lu, J. P. Sculley, D. Yuan, R. Krishna, Z. Wei and H.-C. Zhou, Polyamine-Tethered Porous Polymer Networks for Carbon Dioxide Capture from Flue Gas, Angew. Chem., Int. Ed., 2012, 51(30), 7480–7484 CrossRef CAS PubMed.
- V. Manovic and E. J. Anthony, Steam reactivation of spent CaO-based sorbent for multiple CO2 capture cycles, Environ. Sci. Technol., 2007, 41(4), 1420–1425 CrossRef CAS.
- E. B. Rinker, S. S. Ashour and O. C. Sandall, Absorption of Carbon Dioxide into Aqueous Blends of Diethanolamine and Methyldiethanolamine, Ind. Eng. Chem. Res., 2000, 39(11), 4346–4356 CrossRef CAS.
- M. L. Gray, Y. Soong, K. J. Champagne, J. Baltrus, R. W. Stevens Jr, P. Toochinda and S. S. C. Chuang, CO2 capture by amine-enriched fly ash carbon sorbents, Sep. Purif. Technol., 2004, 35(1), 31–36 CrossRef CAS.
- H. An, B. Feng and S. Su, CO2 capture capacities of activated carbon fibre-phenolic resin composites, Carbon, 2009, 47(10), 2396–2405 CrossRef CAS PubMed.
- J. Wei, J. Shi, H. Pan, Q. Su, J. Zhu and Y. Shi, Thermal and hydrothermal stability of amino-functionalized SBA-16 and promotion of hydrophobicity by silylation, Microporous Mesoporous Mater., 2009, 117(3), 596–602 CrossRef CAS PubMed.
- J.-E. Park, H.-K. Youn, S.-T. Yang and W.-S. Ahn, CO2 capture and MWCNTs synthesis using mesoporous silica and zeolite 13X collectively prepared from bottom ash, Catal. Today, 2012, 190(1), 15–22 CrossRef CAS PubMed.
- Y. Le, D. Guo, B. Cheng and J. Yu, Amine-functionalized monodispersed porous silica microspheres with enhanced CO2 adsorption performance and good cyclic stability, J. Colloid Interface Sci., 2013, 408, 173–180 CrossRef CAS PubMed.
- J. Yu, Y. Le and B. Cheng, Fabrication and CO2 adsorption performance of bimodal porous silica hollow spheres with amine-modified surfaces, RSC Adv., 2012, 2(17), 6784–6791 RSC.
- L. Bastin, P. S. Barcia, E. J. Hurtado, J. A. C. Silva, A. E. Rodrigues and B. Chen, A Microporous Metal–Organic Framework for Separation of CO2/N2 and CO2/CH4 by Fixed-Bed Adsorption, J. Phys. Chem. C, 2008, 112(5), 1575–1581 CAS.
- C. Lu, H. Bai, B. Wu, F. Su and J. F. Hwang, Comparative Study of CO2 Capture by Carbon Nanotubes, Activated Carbons, and Zeolites, Energy Fuels, 2008, 22(5), 3050–3056 CrossRef CAS.
- O. Leal, C. Bolívar, C. Ovalles, J. J. García and Y. Espidel, Reversible adsorption of carbon dioxide on amine surface-bonded silica gel, Inorg. Chim. Acta, 1995, 240(1–2), 183–189 CrossRef CAS.
- Y. Liu, J. Shi, J. Chen, Q. Ye, H. Pan, Z. Shao and Y. Shi, Dynamic performance of CO2 adsorption with tetraethylenepentamine-loaded KIT-6, Microporous Mesoporous Mater., 2010, 134(1–3), 16–21 CrossRef CAS PubMed.
- J. C. Hicks, J. H. Drese, D. J. Fauth, M. L. Gray, G. Qi and C. W. Jones, Designing Adsorbents for CO2 Capture from Flue Gas-Hyperbranched Aminosilicas Capable of Capturing CO2 Reversibly, J. Am. Chem. Soc., 2008, 130(10), 2902–2903 CrossRef CAS PubMed.
- J. Liu, Y. Liu, Z. Wu, X. Chen, H. Wang and X. Weng, Polyethyleneimine functionalized protonated titanate nanotubes as superior carbon dioxide adsorbents, J. Colloid Interface Sci., 2012, 386(1), 392–397 CrossRef CAS PubMed.
- M. G. Plaza, C. Pevida, A. Arenillas, F. Rubiera and J. J. Pis, CO2 capture by adsorption with nitrogen enriched carbons, Fuel, 2007, 86(14), 2204–2212 CrossRef CAS PubMed.
- Q. Ye, J. Jiang, C. Wang, Y. Liu, H. Pan and Y. Shi, Adsorption of Low-Concentration Carbon Dioxide on Amine-Modified Carbon Nanotubes at Ambient Temperature, Energy Fuels, 2012, 26(4), 2497–2504 CrossRef CAS.
- M. B. Yue, L. B. Sun, Y. Cao, Y. Wang, Z. J. Wang and J. H. Zhu, Efficient CO2 capturer derived from as-synthesized MCM-41 modified with amine, Chem.–Eur. J., 2008, 14(11), 3442–3451 CrossRef CAS PubMed.
- Y. G. Ko, S. S. Shin and U. S. Choi, Primary, secondary, and tertiary amines for CO2 capture: designing for mesoporous CO2 adsorbents, J. Colloid Interface Sci., 2011, 361(2), 594–602 CrossRef CAS PubMed.
- N. Hiyoshi, K. Yogo and T. Yashima, Adsorption characteristics of carbon dioxide on organically functionalized SBA-15, Microporous Mesoporous Mater., 2005, 84(1–3), 357–365 CrossRef CAS PubMed.
- X. Xu, C. Song, B. G. Miller and A. W. Scaroni, Influence of Moisture on CO2 Separation from Gas Mixture by a Nanoporous Adsorbent Based on Polyethylenimine-Modified Molecular Sieve MCM-41, Ind. Eng. Chem. Res., 2005, 44(21), 8113–8119 CrossRef CAS.
- S.-C. Hsu, C. Lu, F. Su, W. Zeng and W. Chen, Thermodynamics and regeneration studies of CO2 adsorption on multiwalled carbon nanotubes, Chem. Eng. Sci., 2010, 65(4), 1354–1361 CrossRef CAS PubMed.
- F. Su, C. Lu and H. S. Chen, Adsorption, desorption, and thermodynamic studies of CO2 with high-amine-loaded multiwalled carbon nanotubes, Langmuir, 2011, 27(13), 8090–8098 CrossRef CAS PubMed.
- M. M. Gui, Y. X. Yap, S. P. Chai and A. R. Mohamed, Multi-walled carbon nanotubes modified with (3-aminopropyl)triethoxysilane for effective carbon dioxide adsorption, Int. J. Greenhouse Gas Control, 2013, 14, 65–73 CrossRef CAS PubMed.
- Z.-Z. Zhu, Z. Wang and H.-L. Li, Functional multi-walled carbon nanotube/polyaniline composite films as supports of platinum for formic acid electrooxidation, Appl. Surf. Sci., 2008, 254(10), 2934–2940 CrossRef CAS PubMed.
- H. Gaspar, C. Pereira, S. L. H. Rebelo, M. F. R. Pereira, J. L. Figueiredo and C. Freire, Understanding the silylation reaction of multi-walled carbon nanotubes, Carbon, 2011, 49(11), 3441–3453 CrossRef CAS PubMed.
- C. Baleizão, Vanadyl salen complexes covalently anchored to single-wall carbon nanotubes as heterogeneous catalysts for the cyanosilylation of aldehydes, J. Catal., 2004, 221(1), 77–84 CrossRef PubMed.
- V. Datsyuk, M. Kalyva, K. Papagelis, J. Parthenios, D. Tasis, A. Siokou, I. Kallitsis and C. Galiotis, Chemical oxidation of multiwalled carbon nanotubes, Carbon, 2008, 46(6), 833–840 CrossRef CAS PubMed.
- C. Velasco-Santos, A. L. Martínez-Hernández, M. Lozada-Cassou, A. Alvarez-Castillo and V. M. Castaño, Chemical functionalization of carbon nanotubes through an organosilane, Nanotechnology, 2002, 13(4), 495 CrossRef CAS.
- A. G. Gonçalves, J. L. Figueiredo, J. J. M. Órfão and M. F. R. Pereira, Influence of the surface chemistry of multi-walled carbon nanotubes on their activity as ozonation catalysts, Carbon, 2010, 48(15), 4369–4381 CrossRef PubMed.
- F. Su, C. Lu, W. Cnen, H. Bai and J. F. Hwang, Capture of CO2 from flue gas via multiwalled carbon nanotubes, Sci. Total. Environ., 2009, 407(8), 3017–3023 CrossRef CAS PubMed.
- J. P. Sculley and H.-C. Zhou, Enhancing Amine-Supported Materials for Ambient Air Capture, Angew. Chem., Int. Ed., 2012, 51(51), 12660–12661 CrossRef CAS PubMed.
- J. Liu, A. G. Rinzler, H. Dai, J. H. Hafner, R. K. Bradley, P. J. Boul, A. Lu, T. Iverson, K. Shelimov, C. B. Huffman, F. Rodriguez-Macias, Y.-S. Shon, T. R. Lee, D. T. Colbert and R. E. Smalley, Fullerene Pipes, Science, 1998, 280(5367), 1253–1256 CrossRef CAS.
- R. Sitko, E. Turek, B. Zawisza, E. Malicka, E. Talik, J. Heimann, A. Gagor, B. Feist and R. Wrzalik, Adsorption of divalent metal ions from aqueous solutions using graphene oxide, Dalton Trans., 2013, 42(16), 5682–5689 RSC.
- E. R. Pérez, J. R. Garcia, D. R. Cardoso, B. R. McGarvey, E. A. Batista, U. P. Rodrigues-Filho, W. Vielstich and D. W. Franco, In situ FT-IR and ex situ EPR analysis for the study of the electroreduction of carbon dioxide in N,N-dimethylformamide on a gold interface, J. Electroanal. Chem., 2005, 578(1), 87–94 CrossRef PubMed.
- H. F. Gorgulho, F. Gonçalves, M. F. R. Pereira and J. L. Figueiredo, Synthesis and characterization of nitrogen-doped carbon xerogels, Carbon, 2009, 47(8), 2032–2039 CrossRef CAS PubMed.
- F. Zheng, D. N. Tran, B. J. Busche, G. E. Fryxell, R. S. Addleman, T. S. Zemanian and C. L. Aardahl, Ethylenediamine-Modified SBA-15 as Regenerable CO2 Sorbent, Ind. Eng. Chem. Res., 2005, 44(9), 3099–3105 CrossRef CAS.
- C. Chen, W.-J. Son, K.-S. You, J.-W. Ahn and W.-S. Ahn, Carbon dioxide capture using amine-impregnated HMS having textural mesoporosity, Chem. Eng. J., 2010, 161(1–2), 46–52 CrossRef CAS PubMed.
- M. B. Yue, L. B. Sun, Y. Cao, Z. J. Wang, Y. Wang, Q. Yu and J. H. Zhu, Promoting the CO2 adsorption in the amine-containing SBA-15 by hydroxyl group, Microporous Mesoporous Mater., 2008, 114(1–3), 74–81 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |