Fabrication of NiTe films by transformed electrodeposited Te thin films on Ni foils and their electrical properties
Abstract
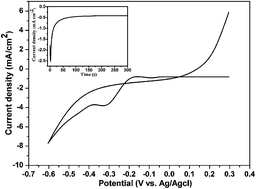
The compact nickel telluride (NiTe) thin film was prepared by two simple steps. High-density tellurium (Te) film with vertical pillar morphology (Te source) was synthesized by a rapid and convenient electrodeposition method without any external agent for the first time. NixTe as the ohmic contact layer material was obtained by a low temperature heat treatment of a Te thin film deposited on a Ni substrate. The structure, morphology, optical and electrical properties of the as-prepared thin films were examined. The current voltage (I–V) characteristic shows that all of the NixTe/Ni samples have already formed ohmic contacts. The NiTe/Ni also shows smaller contact resistance than other samples, which suggests it has potential for application in solar cells as a flexible Ni substrate.

 Please wait while we load your content...
Please wait while we load your content...