Soluble and highly ionically conducting interpolyelectrolyte complexes prepared via chemical template polymerization of aniline in the presence of perfluorinated polysulfonic acid†
Zhanna A. Boeva* and
Vladimir Sergeyev
Lomonosov Moscow State University, Chemistry Department, Polymer Division, Leninskie gory 1 build. 3., Moscow, Russia. E-mail: jboyeva@gmail.com; Tel: +7-4959393117
First published on 27th October 2014
Abstract
Chemical polymerization of aniline in the presence of MF-4SC, a perfluorinated polysulfonic acid, is carried out for the first time in the mixed solvent water/2-propanol. The polymerization results in soluble interpolyelectrolyte complexes forming free-standing films with high proton conductivity, up to 0.05 S cm−1, and electrical conductivity of 6 × 10−5 S cm−1.
Introduction
Mixing aqueous solutions of oppositely charged polyelectrolytes (polymeric acid and polymeric N-base) results in a cooperative electrostatic coupling of the corresponding polyions to form an interpolyelectrolyte complex (IPEC)1 which has a strong potential in the fabrication of novel types of self-assembled materials for various applications.2–4 In the solid state, these complexes can self-organize to form proton conductive pathways.5 In particular, it has been shown that ionically cross-linked membranes of cationic chitosan and anionic poly(acrylic acid) have a high proton conductivity, and an adequate thermal and mechanical stability.6Polyaniline (PANI), in the form of electrically conducting emeraldine salt (ES) is a polycation capable to electrostatic interactions with polyanions.7 The ES form of PANI is insoluble in water and other common organic solvents;8 therefore the traditional method of IPEC preparation is inapplicable. Thus the only way of preparing the IPEC with PANI as a cationic component is a template polymerization of aniline in the presence of an oppositely charged polyelectrolyte. A number of studies have shown that the template polymerization of aniline in aqueous media results in the formation of IPEC which electrical conductivity can be varied from 10−6 up to 1.5 × 10−1 S cm−1.9–14 The internal structure of such composites can be tuned by the synthesis conditions to obtain ultrafine colloids and fibrils for facilitation of the electron transfer in e.g. sensors, transistors and photovoltaic devices.
Perfluorinated polysulfonic acids (PFSA) containing a linear fluorocarbon backbone and fluorinated ether pendant chains terminated with sulfonic acid groups are typical ionomers and can interact with the oppositely charged polyelectrolytes with the formation of IPECs.15 Such IPECs are usually prepared by immersing a perfluorinated ionomer membrane into a polybase solution allowing the polyelectrolyte complexes to form on the surface of the membranes. At the same time, PFSA are usually insoluble in water and therefore the preparation of the IPEC with PANI by mixing or template polymerization in the aqueous media is not applicable and a common solvent has to be found for making that.
In this work we report for the first time that the template polymerization of aniline in the presence of MF-4SC (a representative of the PFSA class of ionomers), can be carried out in a mixed solvent water/2-propanol (10% v/v of water). The template polymerization results in the formation of the IPECs PANI-MF-4SC which is shown by UV-vis and FTIR spectroscopy. The IPECs form free-standing films having proton conductivity which is higher compared to the conductivity of neat MF-4SC. The demonstrated approach of the template chemical polymerization of aniline in the presence of PFSA in the mixed solvents is advantageous for preparing the composite materials when it is difficult to find a common solvent.
Results and discussion
A template-free (ordinary) oxidative polymerization of aniline in water/2-propanol mixture results in a dark-green precipitate. In contrast to that, the polymerization of aniline in the presence of MF-4SC as a template results in a green transparent solution. No precipitate was found at aniline-to-MF-4SC molar ratios (Z) in reaction mixture up to 2.3. The typical evolution of UV-vis spectra of the reaction mixture within the time is shown in Fig. 1. | ||
| Fig. 1 Evolution of UV-vis spectra during the synthesis of PANI-MF-4SC complexes. The experimental conditions: [ANI] = [MF-4SC] = 5 × 10−4 mol L−1, Z = 1, [APS]-to-[ANI] = 1.25. | ||
After an induction period (typically several minutes) the solution turns blue and two absorption peaks at 340 and 620 nm appear in the spectra. As time passing, the intensity of these peaks is increasing and eventually the absorption bands are changing their position in the spectra. Finally the blue colour of the reaction mixture turns green exhibiting a band at around 720 nm. These spectral changes have been described previously and referred to the reduction of the oxidized chains of PANI in the course of the reaction.11 The final spectrum of the reaction mixture 14 hours after the polymerization has absorbance bands at 390 nm corresponding to π–π* transition of benzoid ring and two adsorption peaks at 420 (sh) and 745 nm assigned to polaron band16–19 and referred to emeraldine salt (ES) of PANI in the conformation of compact coil.20 Since ES form of PANI is insoluble in water and other common organic solvents, we assume that the stabilization of PANI in the solution occurs via electrostatic interactions with MF-4SC resulting in the formation of non-stiochiometric IPEC.
According to our experimental data the polymerization of aniline both with and without template does not yield PANI in ES form in the pure 2-propanol, therefore the reaction mixtures additionally have to be diluted with water in order to get 10 vol% of water contents.
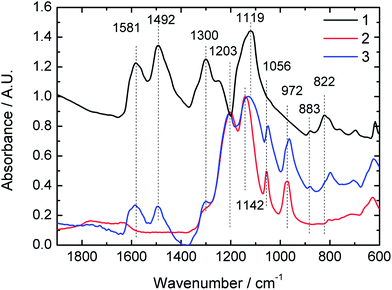
Upon the solution casting the soluble complexes of PANI-MF-4SC form self standing films. The chemical composition of the films was assessed qualitatively with FTIR spectroscopy. The FTIR spectra of the MF-4SC, PANI-MF-4SC films, and polyaniline obtained by the ordinary chemical polymerization are compared in Fig. 2.
The FTIR-spectra of PANI (1) and PANI-MF-4SC composite (3) have characteristic bands at 1581 and 1492 cm−1 attributed to quinoid and benzenoid stretching vibrations of PANI.21,22 Other characteristic bands at 1300, 1250 and 1119 cm−1 in the spectrum of PANI are assigned to Car–N stretching, –C–N˙+ stretching and –NH+= stretching vibrations of PANI in electrically conducting ES form,23,24 correspondingly. In the spectrum of PANI-MF-4SC these peaks are superimposed on the broad CF2 asymmetric stretching vibrations band between 1200 and 1100 cm−1 of MF-4SC.25
As a rule, the infrared spectra of the IPECs can give valuable information about electrostatic interactions between the IPEC components, i.e. between MF-4SC and PANI in case of our study. The spectrum of PANI-MF-4SC contains the bands of symmetric S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of –SO3 groups at 1050 cm−1 and symmetric stretching vibration of the ether side-chain bond (C–O–C) at 960 cm−1 originating from MF-4SC.24 In the spectrum of the IPEC, these bands are shifted towards the higher wavenumbers (1056 and 971 cm−1). Corresponding shifts are typically observed when electrostatic complexation and hydrogen bonding occurs between polyaniline and the functional groups of the template.26 Thus, the results of FTIR analysis clearly prove the formation of polyelectrolyte complex due to template polymerization.
O stretching vibration of –SO3 groups at 1050 cm−1 and symmetric stretching vibration of the ether side-chain bond (C–O–C) at 960 cm−1 originating from MF-4SC.24 In the spectrum of the IPEC, these bands are shifted towards the higher wavenumbers (1056 and 971 cm−1). Corresponding shifts are typically observed when electrostatic complexation and hydrogen bonding occurs between polyaniline and the functional groups of the template.26 Thus, the results of FTIR analysis clearly prove the formation of polyelectrolyte complex due to template polymerization.
It is well known that IPEC formed by polymerization of aniline in the presence of polyanions exhibits rather high electrical conductivity. The DC electrical conductivity of PANI prepared via ordinary chemical polymerization is 2.3 ± 0.4 S cm−1 and is consistent to the previous studies.25 Earlier, it has been reported that the DC conductivity of IPECs of PANI with the aliphatic polysulfonic acids is 10−3 to 10−1 S cm−1.14 However, the electrical conductivity of IPEC formed in the presence of MF-4SC for all studied Z ratios is found to be ca. 6 × 10−5 S cm−1. The above value is 2 orders of magnitude lower than the previously published results. The low electrical conductivity of the IPECs PANI-MF-4SC can be explained by a difference in the supermolecular packing between IPECs having PFSA and linear polyelectrolyte in their composition.
Membranes formed from non-stoichiometric IPECs prepared by simple mixing of negatively and positively charged polyelectrolytes exhibits rather high protonic conductivity.6,27 Additionally, the fluorocarbon backbone of PFSA aggregates in the solution to form macromolecular micelles surrounded by ionic groups and in the solid state these groups form a continuous phase consisting of ionic clusters distributed throughout the perfluorinated hydrocarbon network. Such a phase separation is responsible for the high protonic conductivity of the PFSA,28 and therefore one could be expected also in IPECs PANI-MF-4SC in the solid state.
We have measured the proton conductivity of the PANI-MF-4SC prepared by template polymerization with the different Z ratio with the use of electrochemical impedance spectroscopy (Fig. 3).
As seen, the proton conductivity of the IPECs PANI-MF-4SC obtained at Z ≥ 0.2 decreases exponentially with increasing of the aniline content in reaction mixture. The values are approximately half of those obtained for the neat MF-4SC film (0.02 S cm−1). But interestingly, the IPEC membranes prepared at Z < 0.2 exhibit higher conductivities compared to MF-4SC which is rather rare phenomenon for the composites made of PFSA (e.g. Nafion) and the conducting polymers and can be explained only by the morphological changes occurring on the complexation of MF-4SC with PANI.
As discussed above, in the solution the perfluorinated ionomers form micelles with fluorocarbone phase core surrounded by ionogenic groups. During the template polymerization the PANI chains are organised as compact coils surrounded by ionomer micelles and resulting in the non-stoichiometric IPEC. When the ratio aniline-to-MF-4SC is less than Z < 0.2 in the reaction mixture, the growing chains of PANI acts as bridges connecting the micelles in the larger aggregates, however most of the MF-4SC micelles remain separated. The increase of the amount of aniline in reaction mixture results in the binding more ionomer micelles with PANI and after a certain critical value (presumably, Z = 0.2) the PANI aggregates not only acting as bridges between the micelles but start covering their surface. The mechanism of this process comprises the electrostatic interactions between PANI species and sulfonic acid groups MF-4SC, thus the increase of Z reduces the amount of the free sulfonic acid groups of the MF-4SC micelles responsible for the ion transport within the casted film.
Upon casting MF-4SC forms the films with the structure similar to Nafion membranes in which hydrophobic polymer chains and hydrophilic sulfonic acid groups are phase separated with formation of inverted micelles which ionogenic groups form narrow proton conducting channels and clusters. It has been reported previously that in MF-4SC films quite large amount of these channels are isolated and do not participate in the ion transport in contrast to Nafion membranes where most of the channels and clusters are interconnected.29 Therefore, the high proton conductivity for composite films prepared from Z < 0.2 can be explained by the connection of these separated channels and clusters with PANI species acting as an ionic bridge. When the amount of aniline in the reaction mixture increases, the excess of PANI species expose in conductive channels and contribute to the reduction of ionic conductivity via the complexation of PANI with sulfonic acid groups of MF-4SC. Being involved in the complexation, these groups are getting less hydrated and therefore do not participate in the proton transport, which occurs most efficiently when the sulfonic acid groups of the ionomer are fully hydrated. This explanation well fit to the previously developed ion cluster-channel model,28 according to which a minor changes in the cluster sizes could lead to significant changes in proton conductivity. However this model is not sufficient and further studies are required to verify whether our considerations are reasonable to describe such an interesting phenomenon.
Conclusions
We have shown that soluble interpolyelectrolyte complexes of polyaniline and perfluorinated polysulfonic acid can be prepared by the template polymerization when the common solvent is properly selected. In the mixture of water/2-propanol the polymerization of aniline results in the formation of polyaniline in the electrically conducting emeraldine salt form which is stabilized in the solution by the electrostatic interactions with micelles of MF-4SC. The obtained interpolyelectrolyte complexes form self standing films having electrical conductivity ca. 6 × 10−5 S cm−1 and proton conductivity up to 0.05 S cm−1. The proton conductivity depends on the aniline-to-MF-4SC molar ratio in the initial reaction mixture and decreases upon the increase of polyaniline contents in the complexes. At low aniline contents in the reaction mixture (aniline-to-MF-4SC < 0.2) the proton conductivity of the resulting complexes is found to be higher compared to the neat MF-4SC which can be explained by structural changes of the phase separation mechanism upon casting the films of the complexes. The obtained interpolyelectrolyte complexes have a potential application as membranes for fuel cells and redox batteries as well as photovoltaic and electrochemical devices.Experimental
Materials
Aniline hydrochloride (ANI), and ammonium persulfate (APS) were purchased from SigmaAldrich Co. MF-4SC™ (15 wt% 2-propanol solution) with an equivalent weight (EW) = 860, was the product of JSC Plastpolymer. 2-Propanol (analytical grade, Chimmed) and deionized water, purified with a MilliQ ion-exchange columns system (Millipore), were used as solvents. Hydrochloric acid (HCl, Reachim) for membranes treatment was prepared with dilution of a concentrated HCl (36 wt%, Chimmed) in order to obtain ca. 1 mol L−1 solution.Preparation of polyaniline and polyaniline–PSFA complexes
Template polymerization of aniline was carried out using APS as oxidant in the presence of MF-4SC in water/2-propanol mixture. Typically, 0.1296 g of ANI was dissolved in 10 mL of 2-propanol. The MF-4SC powder obtained by drying the initial solution was dissolved in 2-propanol in order to obtain 8.2 wt% solution. 0.2853 g of APS was dissolved in the mixture of 2.3 mL of bidistilled water and 7.7 mL of 2-propanol as far as it is insoluble in the pure 2-propanol. Solutions of ANI and MF-4SC in 2-propanol were mixed together to produce [ANI]-to-[MF-4SC] (Z) molar ratio 0.01, 0.02, 0.05, 0.1, 0.2, 0.5, 1 and 1.5 and left stirring for 30 min. After that, the solution of APS in the water/2-propanol mixture was added to those mixtures. An [APS]-to-[ANI] molar ratio was maintained 1.25 in each initial reaction mixture. The reaction mixtures were briefly stirred and left to polymerize overnight without stirring at a room temperature (RT).Films from the resulting dispersions of PANI-MF-4SC and the dispersion of MF-4SC in the water/2-propanol mixture were cast onto a flattened glass surface and dried at the RT. The prepared membranes were peeled of the glass and kept (conditioned) in 1 M HCl within 2 hours at 80 °C and the deionized CO2-free water within 2 hours at 80 °C consequently for removal of the polymerization by-products. The conditioned membranes of MF-4SC, PANI-MF-4SC and a powder of PANI were dried first at 40 °C under reduced pressure (10 mbar) for 24 hours, and then were equilibrated at 50% relative humidity under ambient pressure.
Polyaniline doped with the hydrochloric acid was prepared by mixing of aniline hydrochloride solution in 2-propanol with APS solution in water/2-propanol mixture at the same conditions as described above. The resulting black powder was purified with water, acetone and 0.1 M hydrochloric acid. The resulting powder was dried in the air at RT and used as a reference.
Characterization
The UV-vis absorption spectra of the resulting solutions of PANI-MF-4SC were collected with the use of a Fisher Scientific Helios α spectrophotometer.FTIR-ATR spectra of the conditioned and equilibrated at 50% relative humidity samples were collected using a Thermo Nicolet IR 200 instrument equipped with ZnSe ATR performer (incident angle 42°, refractive index 2.4) at 64 interferrograms per spectrum with 8 cm−1 resolution.
The electrical conductivity of the conditioned and equilibrated at 50% relative humidity samples was measured with the use of standard four-point probe technique with 9 V of the applied constant voltage (Loresta). The calculations were done using the finite size model.
Electrochemical impedance spectroscopy measurements were performed using two probes technique with cell arranged with Pt electrodes (surface of electrode is 0.6 cm2) and Autolab potentiostat/galvanostat PGSTAT100. The impedance spectra were collected in galvanostatic mode, within frequency range from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (1 MHz) to 10 Hz, the current was set to be 0.005 A. A piece of membrane soaked in deionized (DI) water was placed between two Pt electrodes and fixed at a constant pressure. The cell with membrane was immersed into beaker of DI water and the impedance spectrum was collected. All the measurements were done at RT and further calculations were carried out according to the protocol described by G. Sherer30 with the use of Frequency Response Analyzer FRA2 software and RQ equivalent circuit.
000 (1 MHz) to 10 Hz, the current was set to be 0.005 A. A piece of membrane soaked in deionized (DI) water was placed between two Pt electrodes and fixed at a constant pressure. The cell with membrane was immersed into beaker of DI water and the impedance spectrum was collected. All the measurements were done at RT and further calculations were carried out according to the protocol described by G. Sherer30 with the use of Frequency Response Analyzer FRA2 software and RQ equivalent circuit.
Acknowledgements
The authors are grateful to the Russian Foundation of Basic Research (project code 11-03-01151), Anthony Guiseppi-Elie for the measurements of electrical conductivity and fruitful discussions, and Sergey A. Chernyak for SEM analysis of the composites.References
- H. F. Mark, Encyclopedia of polymer science and technology: plastics, resins, rubbers, fibers, Interscience Publishers, 1969, vol. 10 Search PubMed.
- R. M. Jisr, H. H. Rmaile and J. B. Schlenoff, Angew. Chem., Int. Ed., 2005, 44, 782–785 CrossRef CAS PubMed.
- T. Groth and A. Lendlein, Angew. Chem., Int. Ed., 2004, 43, 926–928 CrossRef CAS PubMed.
- S. S. Shiratori and M. F. Rubner, Macromolecules, 2000, 33, 4213–4219 CrossRef CAS.
- J. Kerres, A. Ullrich, T. Häring, M. Baldauf, U. Gebhardt and W. Preidel, J. New Mater. Electrochem. Syst., 2000, 3, 129–239 Search PubMed.
- B. Smitha, S. Sridhar and A. A. Khan, Macromolecules, 2004, 37, 2233–2239 CrossRef CAS.
- V. G. Sergeev, N. A. Lokshin, V. B. Golubev, A. B. Zezin, K. Levon and V. A. Kabanov, Dokl. Phys. Chem., 2003, 390, 118–121 CrossRef CAS.
- F. Lux, Polymer, 1994, 35, 2915–2936 CrossRef CAS.
- S. Dorey, C. Vasilev, L. Vidal, C. Labbe and N. Gospodinova, Polymer, 2005, 46, 1309–1315 CrossRef CAS PubMed.
- L. Samuelson and A. Anagnostopoulos, Macromolecules, 1998, 31, 4376–4378 CrossRef CAS.
- Z. A. Boeva, O. A. Pyshkina, A. A. Lezov, G. E. Polushina, A. V. Lezov and V. G. Sergeyev, Polym. Sci., Ser. C, 2010, 52, 35–43 CrossRef.
- G. P. Shumakovich, I. S. Vasil'eva, O. V. Morozova, V. G. Khomenkov, I. N. Staroverova, I. A. Budashov, I. N. Kurochkin, J. A. Boyeva, V. G. Sergeyev and A. I. Yaropolov, J. Appl. Polym. Sci., 2010, 117, 1544–1550 CAS.
- J.-M. Liu and S. C. Yang, J. Chem. Soc., Chem. Commun., 1991, 1529–1531 RSC.
- Z. A. Boeva, O. A. Pyshkina and V. G. Sergeev, Polym. Sci., Ser. A, 2012, 54, 614–620 CrossRef CAS.
- Y. Yang, A. Siu, T. J. Peckham, and S. Holdcroft, in Fuel Cells I, ed. G. G. Scherer, Springer Berlin Heidelberg, Berlin, Heidelberg, 2008, pp. 55–126 Search PubMed.
- P. Ghosh, S. K. Siddhanta and A. Chakrabarti, Eur. Polym. J., 1999, 35, 803–813 CrossRef CAS.
- E. M. Geniès, A. Boyle, M. Lapkowski and C. Tsintavis, Synth. Met., 1990, 36, 139–182 CrossRef.
- O. P. Dimitriev and N. V. Lavrik, Synth. Met., 1997, 90, 1–4 CrossRef CAS.
- D. Stilwell and S. Park, J. Electrochem. Soc., 1989, 136, 427–433 CrossRef CAS PubMed.
- A. Mirmohseni and G. G. Wallace, Polymer, 2003, 44, 3523–3528 CrossRef CAS.
- Y. Wei, K. F. Hsueh and G. W. Jang, Macromolecules, 1994, 27, 518–525 CrossRef CAS.
- J. Tang, X. Jing, B. Wang and F. Wang, Synth. Met., 1988, 24, 231–238 CrossRef CAS.
- J. Ostrowska and A. Narebska, Colloid Polym. Sci., 1983, 261, 93–98 CAS.
- M. Trchova, J. Stejskal and J. Prokeš, Synth. Met., 1999, 101, 840–841 CrossRef CAS.
- A. Gruger, A. Régis, T. Schmatko and P. Colomban, Vib. Spectrosc., 2001, 26, 215–225 CrossRef CAS.
- J. Stejskal and R. G. Gilbert, Pure Appl. Chem., 2002, 74, 857–867 CrossRef CAS.
- J. Kerres, A. Ullrich, T. Häring, M. Baldauf, U. Gebhardt and W. Preidel, J. New Mater. Electrochem. Syst., 2000, 3, 129–239 Search PubMed.
- K. A. Mauritz and R. B. Moore, Chem. Rev., 2004, 104, 4535–4586 CrossRef CAS.
- S. S. Ivanchev and S. V. Myakin, Russ. Chem. Rev., 2010, 79, 101–117 CrossRef CAS PubMed.
- L. Gubler, N. Prost, S. Gursel and G. Scherer, Solid State Ionics, 2005, 176, 2849–2860 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Scanning Electron Microscopy (SEM) images of MF-4SC film, and PANI-MF-4SC 1.5 and PANI-MF-4SC 0.1 films. See DOI: 10.1039/c4ra11217b |
| This journal is © The Royal Society of Chemistry 2014 |