Facile synthesis of smart biopolymeric nanofibers towards toxic ion removal and disinfection control
Abstract
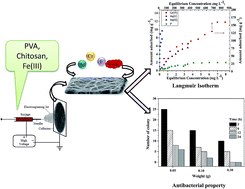
To provide safe drinking water, it is crucial to tackle both bacterial infection and inorganic pollutants. In this study, smart biopolymeric nanofibers consisting of chitosan/Fe(III)/PVA (CPF) have been synthesized via an electrospinning method. Crosslinking with glutaraldehyde was carried out to increase the stability of the mats. The prepared CPF mat exhibited increased adsorption sites, facilitating the removal of As(III), As(V), Cr(VI) and F− ions. The selectivity order of the investigated anions towards CPF mats followed the sequence: Cr(VI) > As(V) > As(III) > F. The maximum adsorption capacities of Cr(VI), As(V), As(III) and F were found to be 166.7, 83.3, 32.3 and 2.5 mg g−1, respectively. The prepared CPF mat also exhibited 100% disinfection towards E. coli at an initial concentration of 104–105 CFU ml−1. Using FTIR studies, the mechanism of interaction of these ions with CPF has also been postulated.

 Please wait while we load your content...
Please wait while we load your content...