Renal-specific delivery of prednisolone-folate conjugates for renal ischemia/reperfusion injury†
Qin Zhang‡
a,
Yao Fu‡a,
Renhe Liub,
Tao Gonga,
Xun Suna and
Zhi-Rong Zhang*a
aKey Laboratory of Drug Targeting & Drug Delivery Systems Ministry of Education, West China School of Pharmacy Sichuan University, No. 17, Section 3, Renmin Nan Rd, Chengdu, 610041, China. E-mail: zrzzl@vip.sina.com
bDepartment of Chemistry, The Scripps Research Institute, 10550 N. Torrey Pines Road, La Jolla, CA 92037, USA
First published on 3rd October 2014
Abstract
Prednisolone-folate conjugate (PFC) was synthesized to achieve renal-targeted delivery and specific intracellular release of prednisolone. Our results highlight the significance of folate-mediated targeted delivery to the kidney and the consequent in vivo therapeutic efficacy of PFC against renal ischemia/reperfusion injury.
Renal ischemia/reperfusion injury (IRI) commonly occurs in kidney transplantation, cardiopulmonary and aortic bypass surgery, trauma, hemorrhage, hypotension and burns, which affect a large population of patients worldwide.1–3 As a leading cause of acute renal failure, renal IRI is associated with very high morbidity and mortality.4 Glucocorticoids (GCs) are a class of steroid hormones that have been successfully used in clinic for their anti-inflammatory and immunosuppressive effects.5 GCs have shown reno-protective effects against renal IRI by a receptor-dependent, non-genomic mechanism in the rat renal IRI model.5–7 GCs exert effects by entering target cells and binding to the inactive cytoplasmic glucocorticoid receptors. The activated ligand–receptor complex then translocates into the nucleus to regulate gene transcription either directly or indirectly.8,9 However, extensive and severe adverse effects were reported for GCs when administered systemically mainly due to a non-specific distribution of GCs in vivo, including osteoporosis, peptic ulcer, hyperglycemia and weight gain.10,11 Thus, a renal specific delivery of GCs would be beneficial to improve their therapeutic efficacy against renal IRI while limiting systemic adverse effects.
Our lab has focused on the development of renal-specific delivery systems for years using prednisolone as the selected therapeutic molecule. Previously, several strategies have been explored to deliver prednisolone selectively to the kidney, e.g., N-acetylated low molecular weight chitosan–prednisolone conjugates, prednisolone–glucose, and prednisolone–glucosamine conjugates which showed significantly higher intrarenal drug concentration compared to the free drug and the specificity to proximal tubule epithelial cells (PTECs).12–16 These glycoconjugates were designed to achieve renal targeting attributed to the specific interaction between sugar moieties and glucose transporters or megalin/cubilin receptors that are extensively distributed in the renal proximal tubules. However, extensive distribution of glucose and other related transporters throughout the human body present potential challenges on the targeted delivery of prednisolone to kidney via glycoconjugates. Therefore, more specific delivery strategies need to be developed to overcome existing challenges. In general, a successful renal-specific delivery system requires: (i) an efficient renal-targeting moiety, e.g., ligands that can interact with specific receptors in the kidney; (ii) good aqueous solubility that favors rapid distribution to the kidney via systemic administration; (iii) a suitable linker that is stable in the blood circulation and can further be cleaved to achieve drug release at the target site.
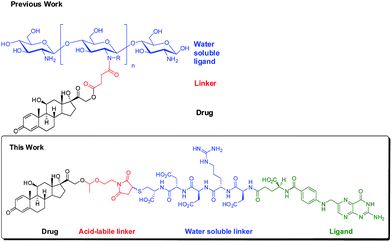
Herein, we report a new prednisolone conjugate candidate derived from prednisolone and folate to selectively deliver prednisolone to PTECs in the kidney. High folate receptor-α expression was well demonstrated in the PTECs thus making it an excellent target for specific drug delivery to the kidney.17,18 Previously, folate-conjugated agents for tumor imaging were shown to distribute specifically in the kidney in addition to the tumor site.19,20 This is mostly likely due to a relatively high level of folate receptor-α expression in PTECs. Moreover, the re-absorption of filtered solutes by PTECs may also contribute to the accumulation of folate conjugates in the kidney. However, tumor-targeted folate conjugates did not display nephrotoxicity which implies that folate conjugates might undergo rapid exocytosis and might be further transported across the basolateral membrane and into the circulation.19,21 Folate conjugates are suggested to enter cells via receptor-mediated endocytosis and undergo stages of endosomal and lysosomal metabolism and degradation in the cytoplasm. Folate receptor-containing endosomal pH was proven around 6.5 which is critical to the intracellular release of parent drug.22 Based on these findings, an acid-labile acetal linker was introduced in the design of prednisolone-folate conjugate (PFC) to allow specific cleavage and activation of the prodrug in the endosomes of PTECs. The structure of acetals offers a reasonable stability under extracellular conditions while undergoing fast hydrolysis in an acidic milieu.23 Derived from previous studies,20,24,25 the proposed PFC structure consists of a water soluble pentapeptide spacer rendering the conjugate more water-soluble, a folic acid moiety with targetability to PTECs in the kidney, an acid-labile linker that allows the intracellular release of drug and the therapeutic moiety of prednisolone (Fig. 1). A straight forward synthetic strategy was thus developed in the study to synthesize PFC (Scheme S1, ESI†).
To confirm cleavage and release of prednisolone under mildly acidic conditions, the in vitro stability of PFC was evaluated at varying pH conditions. PFC showed an aqueous solubility of about 3.27 mg mL−1 in the neutral buffer saline while prednisolone was reported to be nearly insoluble in water. At 37 °C, 99% of PFC remained unchanged for 29 h. Also, PFC remained stable in the rat plasma with 90% of the conjugates remaining unchanged for 6 h and 85% of the conjugates remaining unchanged for 12 h (Fig. S1, ESI†). Maintaining plasma stability is critical to the successful delivery of the conjugate to the target organ through systemic administration without encountering quick hydrolysis or degradation in the plasma. At pH 5, less than 50% of the conjugate was hydrolyzed by 12 h, while at pH 2, over 60% of the conjugate was hydrolyzed by 4 h (Fig. S2, ESI†). Thus, the acid-labile property of the conjugate would trigger the specific release of prednisolone in the mildly acidic endosomes via folate-receptor mediated endocytosis while remaining stable in the circulation.
To gain insight into the in vivo profiles of the PFC conjugate, the pharmacokinetic profiles of PFC and prednisolone were evaluated in rats. Both PFC and prednisolone displayed a profile with decreasing plasma concentration over time in vivo (Fig. 2). Interestingly, PFC displayed significantly higher plasma concentrations at all time points than prednisolone (p < 0.05). In contrast to prednisolone, a 7.7-fold higher AUC0–t value and a 1.9-fold higher mean-residence-time (MRT0–t) value were observed for PFC. Pharmacokinetic parameters, including maximum plasma concentration (Cmax), relative uptake efficiency (Rekidney) and concentration efficiency (Cekidney) of PFC and prednisolone in kidney, are presented in Table S2 (ESI†).
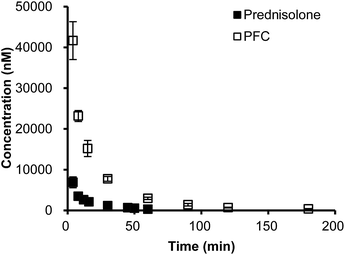 | ||
| Fig. 2 The time-concentration profile of prednisolone in plasma after injection of prednisolone and prednisolone-folate conjugate (PFC). Data represent mean ± S.D. (n = 3). | ||
Regarding kidney bioavailability (Fig. 3), PFC displayed significantly higher levels of distribution in the rat kidney compared with free prednisolone over the course of investigation (p < 0.05). Compared to previously reported prednisolone conjugate systems, a remarkably higher Rekidney of 116.83 was observed for PFC (Table S3, ESI†), while a Rekidney of 5.64 was reported for prednisolone–glucosamine system,26 which provided strong evidence for selecting folate as the targeting ligand for specific renal drug delivery.
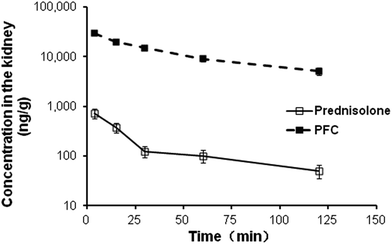 | ||
| Fig. 3 The concentration–time profile of prednisolone and PFC in rat kidney after intravenous injection (equivalent to 3 mg kg−1 prednisolone). Data represent mean ± S.D., (n = 5). | ||
To further evaluate the kidney targetability of PFC, the tissue distribution profile of PFC and prednisolone was measured at 4 and 30 min after i.v. injection (Fig. 4). At 4 min, PFC displayed significantly higher distributions in both kidney and plasma than in other organs, while concurrently prednisolone showed higher distributions in intestine and pancreas. At 30 min, PFC maintained the specific distribution in the kidney with a much higher concentration compared to all other organs. However, PFC also displayed increased distribution in the intestine at 30 min after single i.v. injection, a phenomenon that has been previously reported.25 The increase in the intestinal distribution may be due to the folate receptor expression in the intestine. Additionally, promiscuous organic anion transporters (OATP) may also contribute to the transport of folate conjugates in the intestine. Studies showed folate conjugates avoided being recognized and transported by OATP through increasing the steric hindrance of the linker linking folate and therapeutic agent.27
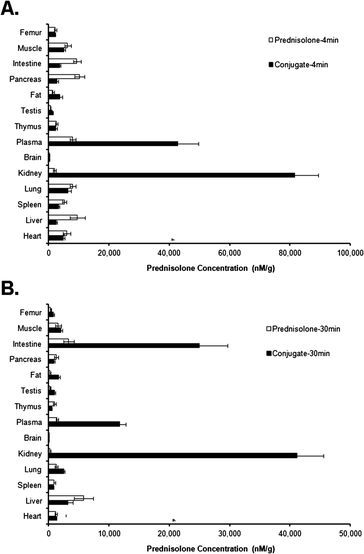 | ||
| Fig. 4 Tissue distribution in rats 4 min (A) and 30 min (B) after i.v. injection of prednisolone (white columns) and PFC (black columns). Data represent mean ± SD, (n = 5). | ||
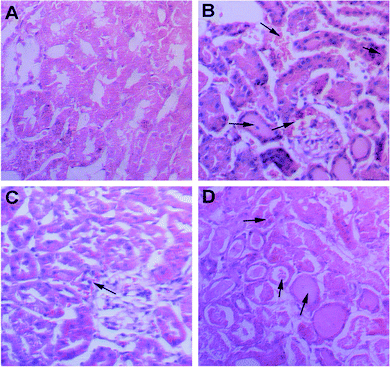
To investigate the reno-protective effect of PFC against renal IRI in rats, a rat renal IRI model was established with some modifications to the previous study.28 PFC, which was consecutively given at 3 mg kg−1 for three days, effectively prevented and alleviated acute injury to renal tubules by maintaining serum creatine (CRER) and blood urea nitrogen (BUN) at normal levels compared to the sham group (Table S4, ESI†). Furthermore, the renal morphology of ischemia/reperfusion (I/R) group displayed common renal damages such as dilatation of the tubular lumen, degeneration of the renal tubular epithelial cells, infiltration of leukocytes, and formation of protein casts (Fig. 5B). Compared with I/R group, renal damages were relieved to a certain extent in the I/R group treated with prednisolone, though dilation of the tubular lumen, degeneration of tubular epithelia cells and protein casts were observed (Fig. 5D). Only mild degeneration of the renal tubular cells, dilatation of the tubular lumen and few casts were observed in the I/R group treated with PFC compared to the sham-operated group (Fig. 5A and C), which demonstrated a better therapeutic performance for PFC in vivo and thus a great potential in renal IRI treatment.
In summary, we utilized folate as the targeting ligand to achieve renal-specific delivery of prednisolone. PFC was demonstrated to have improved water solubility, plasma stability, and acid-sensitive properties that are crucial to achieving targeted kidney delivery via systemic administration. Moreover, PFC displayed excellent renal targetability in vivo, which was also proven to successfully reverse the disease progression in the rat renal I/R model indicating that PFC can serve as a potential prodrug candidate for renal IRI therapy.
Acknowledgements
This work was supported by the National S&T Major Project of China (Grant no. 2011ZX09310-002) and the National Natural Science Foundation of China (no. 81130060).Notes and references
- Z. Aydin, A. J. van Zonneveld, J. W. de Fijter and T. J. Rabelink, Nephrol., Dial., Transplant., 2007, 22, 342–346 CrossRef PubMed
.
- C. M. Mangano, L. S. Diamondstone, J. G. Ramsay, A. Aggarwal, A. Herskowitz and D. T. Mangano, The Multicenter Study of Perioperative Ischemia Research Group, Ann. Intern. Med., 1998, 128, 194–203 CrossRef CAS
.
- A. Kazmers, L. Jacobs and A. Perkins, J. Surg. Res., 1997, 67, 62–66 CrossRef CAS PubMed
.
- G. M. Chertow, E. Burdick, M. Honour, J. V. Bonventre and D. W. Bates, J. Am. Soc. Nephrol., 2005, 16, 3365–3370 CrossRef PubMed
.
- S. Kumar, D. A. Allen, J. E. Kieswich, N. S. A. Patel, S. Harwood, E. Mazzon, S. Cuzzocrea, M. J. Raftery, C. Thiemermann and M. M. Yaqoob, J. Am. Soc. Nephrol., 2009, 20, 2412–2425 CrossRef CAS PubMed
.
- K. Salmela, L. Wramner, F. Ekberg, I. Hauser, O. Bentdal, L. E. Lins, H. Isoniemi, L. Backman, N. Persson, H. H. Neumayer, P. F. Jorgensen, C. Spieker, B. Hendry, A. Nicholls, G. Kirste and G. Hasche, Transplantation, 1999, 67, 729–736 CrossRef CAS PubMed
.
- A. Reutzel-Selke, T. Zschockelt, C. Denecke, U. Bachmann, A. Jurisch, J. Pratschke, G. Schmidbauer, H. D. Volk, P. Neuhaus and S. G. Tullius, Transplantation, 2003, 75, 1786–1792 CrossRef CAS PubMed
.
- C. Stahn, M. Loewenberg, D. W. Hommes and F. Buttgereit, Mol. Cell. Endocrinol., 2007, 275, 71–78 CrossRef CAS PubMed
.
- H. Schacke, A. Schottelius, W. D. Docke, P. Strehlke, S. Jaroch, N. Schmees, H. Rehwinkel, H. Hennekes and K. Asadullah, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 227–232 CrossRef PubMed
.
- H. Schacke, W. D. Docke and K. Asadullah, Pharmacol. Ther., 2002, 96, 23–43 CrossRef CAS
.
- E. Bakina, Z. Wu, M. Rosenblum and D. Farquhar, J. Med. Chem., 1997, 40, 4013–4018 CrossRef CAS PubMed
.
- Z.-X. Yuan, X. Sun, T. Gong, H. Ding, Y. Fu and Z.-R. Zhang, J. Drug Targeting, 2007, 15, 269–278 CrossRef CAS PubMed
.
- Z.-X. Yuan, Z.-R. Zhang, D. Zhu, X. Sun, T. Gong, J. Liu and C.-Y. Luan, Mol. Pharmaceutics, 2009, 6, 305–314 CrossRef CAS PubMed
.
- Z.-X. Yuan, J.-J. Li, D. Zhu, X. Sun, T. Gong and Z.-R. Zhang, J. Drug Targeting, 2011, 19, 540–551 CrossRef CAS PubMed
.
- Y. Lin, Y. P. Li, X. H. Wang, T. Gong, L. Zhang and X. Sun, J. Controlled Release, 2013, 167, 148–156 CrossRef CAS PubMed
.
- X. Liu, W. Li, Z. Liang, X. Zhang, Y. Guo, T. Gong and Z. Zhang, Arch. Pharm., 2012, 345, 925–933 CrossRef CAS PubMed
.
- H. Birn, O. Spiegelstein, E. I. Christensen and R. H. Finnell, J. Am. Soc. Nephrol., 2005, 16, 608–615 CrossRef CAS PubMed
.
- B. A. Kamen and A. K. Smith, Adv. Drug Delivery Rev., 2004, 56, 1085–1097 CrossRef CAS PubMed
.
- C. P. Leamon, J. A. Reddy, I. R. Vlahov, P. J. Kleindl, M. Vetzel and E. Westrick, Bioconjugate Chem., 2006, 17, 1226–1232 CrossRef CAS PubMed
.
- C. P. Leamon, M. A. Parker, I. R. Vlahov, L. C. Xu, J. A. Reddy, M. Vetzel and N. Douglas, Bioconjugate Chem., 2002, 13, 1200–1210 CrossRef CAS PubMed
.
- C. P. Leamon, J. A. Reddy, I. R. Vlahov, E. Westrick, A. Dawson, R. Dorton, M. Vetzel, H. K. Santhapuram and V. Wang, Mol. Pharmaceutics, 2007, 4, 659–667 CrossRef CAS PubMed
.
- J. Yang, H. Chen, I. R. Vlahov, J.-X. Cheng and P. S. Low, J. Pharmacol. Exp. Ther., 2007, 321, 462–468 CrossRef CAS PubMed
.
- A. Schlossbauer, C. Dohmen, D. Schaffert, E. Wagner and T. Bein, Angew. Chem., Int. Ed., 2011, 50, 6828–6830 CrossRef CAS PubMed
.
- J. M. Shillingford, C. P. Leamon, I. R. Vlahov and T. Weimbs, J. Am. Soc. Nephrol., 2012, 23, 1674–1681 CrossRef CAS PubMed
.
- S. F. Knight, K. Kundu, G. Joseph, S. Dikalov, D. Weiss, N. Murthy and W. R. Taylor, J. Am. Soc. Nephrol., 2012, 23, 793–800 CrossRef CAS PubMed
.
- Y. Lin, Y. Li, X. Wang, T. Gong, L. Zhang and X. Sun, J. Controlled Release, 2013, 167, 148–156 CrossRef CAS PubMed
.
- C. P. Leamon, J. A. Reddy, P. J. Klein, I. R. Vlahov, R. Dorton, A. Bloomfield, M. Nelson, E. Westrick, N. Parker, K. Bruna, M. Vetzel, M. Gehrke, J. S. Nicoson, R. A. Messmann, P. M. LoRusso and E. A. Sausville, J. Pharmacol. Exp. Ther., 2011, 336, 336–343 CrossRef CAS PubMed
.
- Z. Zhang, Q. Zheng, J. Han, G. Gao, J. Liu, T. Gong, Z. Gu, Y. Huang, X. Sun and Q. He, Biomaterials, 2009, 30, 1372–1381 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental methods, characterization data. See DOI: 10.1039/c4ra11160e |
| ‡ These authors contributed equally to this research. |
| This journal is © The Royal Society of Chemistry 2014 |