A low-cost and rapid microfluidic paper-based analytical device fabrication method: flash foam stamp lithography
Abstract
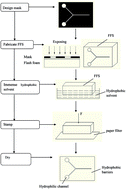
A novel and facile fabrication method of microfluidic paper-based analytical devices (μPADs) with flash foam stamp lithography (FFSL) is presented in this paper. First, a flash foam (also called photosensitive seal) stamp with desired patterns is made by flash exposing. Next, the stamp is immersed in a hydrophobic solvent such as polydimethylsiloxane (PDMS) to absorb the ink. Finally, the hydrophobic solvent is stamped on filter paper to form hydrophobic barriers. After the hydrophobic solvent cures, the μPAD is complete. Compared to common fabrication methods such as wax printing, inkjet printing, or direct writing, this paper will demonstrate that the FFSL method is convenient, quick, and cheap.

 Please wait while we load your content...
Please wait while we load your content...