Highly efficient carbon catalyzed aerobic selective oxidation of benzylic and allylic alcohols under transition-metal and heteroatom free conditions†
Abstract
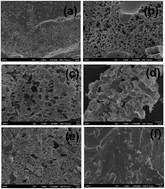
The aerobic oxidation of benzylic and allylic alcohols has been realized with carbon material as the catalyst in the absence of transition-metal and heteroatoms. Under the optimized reaction conditions, aldehyde and acid can be selectively synthesized with up to 99% yields. The carbon catalyst can be easily recovered from the reaction mixture and can be reused for 5 runs without deactivation.

 Please wait while we load your content...
Please wait while we load your content...