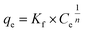DOI:
10.1039/C4RA11016A
(Paper)
RSC Adv., 2015,
5, 784-790
Study of chromium(VI) removal from aqueous solution using nitrogen-enriched activated carbon based bamboo processing residues
Received
23rd September 2014
, Accepted 3rd November 2014
First published on 4th November 2014
Abstract
Nitrogen functional groups were introduced by urea and melamine onto the surface of two bamboo processing residues derived activated carbons (ACs) and Cr(VI) adsorption was investigated by changing various parameters. The results suggested that the incorporation of nitrogen species caused a visible increase in the adsorption capacity. The ACs with melamine and urea modification showed that the maximum removal of Cr(VI) from the solution having an initial Cr(VI) concentration of 100 mg L−1 was obtained at pH 2.0 as 89% and 85%, respectively. The adsorption capacity of Cr(VI) for unmodified AC was 78%. Langmuir adsorption model was applied to experimental equilibrium data of Cr(VI) adsorption and the adsorption kinetic followed pseudo-second-order model for these two ACs. Besides, the intraparticle diffusion kinetic model suggested the Cr(VI) adsorption could be divided into two phases: the diffusion controlled by external surface followed by an intra-particle diffusion.
Introduction
The increasing contamination of urban and industrial wastewaters by toxic metal ions causes important environmental pollutions. These inorganic micro-pollutants are of considerable concern because they are non-biodegradable, highly toxic and have a probable carcinogenic effect.1 Chromium (Cr) is one of the most toxic and harmful heavy metals which is generated from various industrial processes such as: leather tanning, electroplating, manufacturing of dye and paint, pulping and papermaking, and the petroleum refining.2 The maximum contaminant limit of chromium in drinking water is 100 μg L−1 if according to the standard of the United States Environmental Protection Agency, or 50 μg L−1 if according to a more strict standard of the World Health Organization. The mobility, solubility, and toxicity of chromium are highly dependent on the oxidation state of the metal. Just in 2011 the production of the FeCr2O4 was 23.3 million tons in the world.3 And if water contains a high concentration of Cr may cause serious environmental problems as well as induce toxic and carcinogenic health problems on humans. As you known, Cr(VI) is 500 times more toxic than the Cr(III) form. Due to its high toxicity, the removal of Cr(VI) from wastewaters arouses great attention.4
Activated carbons (ACs) can be a good alternative for the removal of Cr(VI), which is a high-porosity material, very useful in the adsorption of both gases and solutes. Therefore, it has been widely used for the separation of gases, recovery of solvents and removal of organic pollutants inorganic micropollutants from drinking water, as well as a catalyst carbon.5 However, the cost of generation and regeneration of activated carbon is relatively high, for this reason, the production of low cost, disposable sorbents for removal Cr(VI) is worth considering. Recently, agricultural by-products have received an increasing attention for the production of activated carbons due to their low-cost, renewability and wide prevalence.6 In China, bamboo is widely planted and spread, there are approximately 400 species of bamboo in 35 genera, which is one third of the total number of species in the world. Bamboo has become a renewable organic resource for sustainable development and has been used in construction, clothing, household appliances and entertainment materials to make different products. However, the bamboo residues coming from the bamboo manufacturing factory are often dumped or burnt as wastes, which results in environmental pollution and waste of resources.
Therefore, the conversion of bamboo processing residues into activated carbons (ACs) would add considerable economic value, help reduce the cost of waste disposal and most importantly, provide a potentially inexpensive alternative to the existing commercial ACs.
Recently, nitrogen containing ACs are subjects of particular interest for researchers because they have shown a high versatility and efficiency in processing of waste products (in both gas and liquid phase, such as CO2, NOx, H2S, SO2, CH4, heavy metals and a wide range of organic compounds).7
In this work, two N-enriched bamboo residues based AC materials modified with urea and melamine were developed, The adsorbents are characterized by elemental analysis, X-ray diffraction (XRD), and nitrogen adsorption analyses for Brunauer–Emmett–Teller (BET) specific surface area, and then tested in comparison to AC without modification for Cr(VI) removal. The Cr(VI) adsorption kinetics and adsorption isotherms were performed to demonstrate the superior activity of the new materials. The influence of several operating parameters, such as contacting time, pH of solution and Cr(VI) initial concentration were investigated in batch system.
Experiments and materials
Materials
Bamboo waste was kindly provided by Nanjing Forestry University; urea and melamine as nitrogen (N) source for synthesis of N-enriched AC were analytical and were purchased from Kebaiao Chemical reagent, other chemicals were analytical grades and were purchased from Beijing Lanyi Chemical reagent. Double distilled water was used for preparation of all required solutions.
Preparation of Acs
Three AC samples were obtained by the following process. Carbonization was carried out in nitrogen atmosphere at the temperature increase rate of 10 °C min−1 to the final temperature of 500 °C, separately, maintained for 1 h. The carbonized sample were then ground and screened out with sieves. The fraction in the particle diameter ranged from 40 meshes to 60 meshes. The particle samples were dried in a 105 °C oven for 8 h; in activation process, 3 grams of the oven-dried samples were mixed with melamine or urea at the ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, the resulting mixture were soaked in a 50% K2CO3 solution for 16 h at the mass ratio impregnation (3
3, the resulting mixture were soaked in a 50% K2CO3 solution for 16 h at the mass ratio impregnation (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The samples were then activated at 800 °C for 60 min in nitrogen atmosphere. The obtained ACs modified with melamine and urea were marked AC2 and AC3, respectively, and the controlled sample activated at 800 °C for 60 min in nitrogen atmosphere with K2CO3 at the mass ratio of (3
1). The samples were then activated at 800 °C for 60 min in nitrogen atmosphere. The obtained ACs modified with melamine and urea were marked AC2 and AC3, respectively, and the controlled sample activated at 800 °C for 60 min in nitrogen atmosphere with K2CO3 at the mass ratio of (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) without modification was marked AC1. After carbonization and activation process, all samples were boiled first with 1 M HCl solution and then washed with distilled water until the pH of solution reach to about 6–7. Finally, these ACs were dried at 105 °C in an oven for 6–8 h.
1) without modification was marked AC1. After carbonization and activation process, all samples were boiled first with 1 M HCl solution and then washed with distilled water until the pH of solution reach to about 6–7. Finally, these ACs were dried at 105 °C in an oven for 6–8 h.
Characterization of the Acs
Chemical surface composition. Chemical surface composition and state of the samples were determined by X-ray photoelectron spectroscopy (XPS), elemental analysis and FT-IR. (i) X-ray was performed on an ESCALAB250 (VGScientific, UK) using a monochromatic Al Kα radiation. The acceleration tension and power of X-ray source were 15 kV and 100 W, respectively. The sample charging was corrected by using the C1s peak (284.6 eV) as an internal standard. (ii) The elemental analysis (contents in carbon, hydrogen, and nitrogen) of the activated carbons was under taken in a CHNS Analyzer (Thermofinnigan Flash, EA, 1112 series). (iii) FTIR spectra of the carbon samples were obtained with a FTIR spectrum spectrometer (PerkinElmer, spectrum100d). The activated carbon–KBr mixtures (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) were ground, and then desorbed at room temperature and pressed to obtain IR-transparent pellets. The measurements were carried out over 4000–400 cm−1.
100) were ground, and then desorbed at room temperature and pressed to obtain IR-transparent pellets. The measurements were carried out over 4000–400 cm−1.
Porous texture. The N2 adsorption–desorption isotherms of ACs were measured with an accelerated surface area and porosimetry system (ASAP 2010, Micromeritics) for determining the surface areas. Prior to the measurements, the samples were outgassed at 573 K, under nitrogen flow for at least 2 h. The nitrogen adsorption–desorption data were recorded at a liquid nitrogen temperature of 77 K. The nitrogen adsorption isotherm was measured over a relative pressure (p/p0) range, from approximately 10−6 to 1. The BET surface area was calculated using the BET equation from the selected N2 adsorption data, within a range of relative pressure, p/p0, from 0.1 to 0.3. Pore size distribution in the micropore range was obtained by the Barrett–Joyner–Halenda (BJH) method.8
Cr(VI) adsorption experiments
Batch Cr(VI) adsorption experiments were carried out by using the ACs as the adsorbents. To start the experiment, a known amount of adsorbent was introduced to aqueous solution with a desired concentration of Cr(VI). The flasks were shaken under 200 rpm for 24 h at desired temperatures to ensure that the sorption process reached equilibrium. The pH of the solutions was regulated by micro-additions of 0.1 M hydrochloric acid (HCL) and 0.1 M sodium hydroxide (NaOH). For the adsorption experiment, the concentration of Cr(VI) was determined by using UV absorption in a UV-2102C spectrophotometer (Unico, USA) with a wavelength of max 540 nm.9 The percentage of the removed Cr(VI) was calculated using eqn (1):| |
 | (1) |
where Co and Ce (mg L−1) are the liquid-phase concentration of Cr(VI) at initial and equilibrium, respectively. The amount of Cr(VI) adsorbed q in (mg g−1) was calculated using eqn (2):| |
 | (2) |
where q is the Cr(VI) uptake at equilibrium (mg g−1), m is the mass of the ACs used (g) and V is the volume of the solution (L).10
Results and discussion
Characteristics of the adsorbent
C, H, O, N contents of the ACs prepared from bamboo residues were shown in Table 1, respectively. As can be seen from Table 1, compared with the AC1, AC2 and AC3 had higher N content, it means that after activation process, the nitrogen groups in the modifiers were introduced onto the surface of ACS. The AC2 modified with melamine contained 1.34 times higher of N than that of AC3 modified with urea. The corresponding thing is that the content of N in the melamine is almost 1.34 times higher than that of in urea, which indicates that the more N in the modifier, the more N remained in the ACs.
Table 1 Elemental analysis of the ACs
| ACs prepared from bamboo waste |
Elemental analysis |
| O (wt%) |
C (wt%) |
H (wt%) |
N (wt%) |
| AC1 |
6.05 |
90.59 |
0.524 |
0.491 |
| AC2 |
4.59 |
86.81 |
1.082 |
6.269 |
| AC3 |
4.78 |
87.54 |
0.819 |
4.717 |
XPS study
Fig. 1 showed the complex XPS N1s spectra of ACs containing N. The peaks were assigned to different forms of N atoms substituted for carbon in graphene layer: N-6, N-5, N-Q and N-X, respectively.11 Hence, N-6 (398.7 ± 0.3 eV) was pyridinic-N, i.e. N bonded to two carbon atoms in six membered rings at the edge of graphene layer. N-5 (400.3 ± 0.3 eV) represented pyrrolic-N in five-membered ring and/or pyridonic-N. N-Q (401.4 ± 0.4 eV) corresponded to quaternary-N, i.e. nitrogen bonded to three carbon atoms in central or valley position of graphene layer. Finally, N-X (402–405 eV) was pyridine N-oxide.12,13
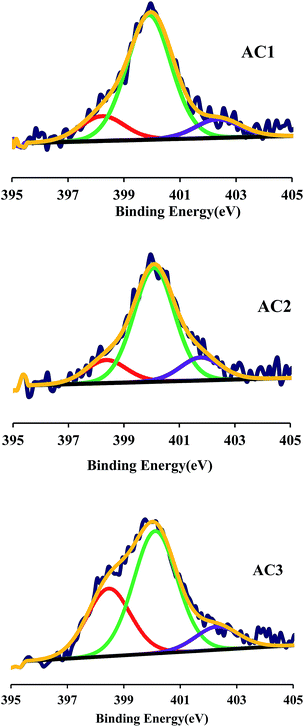 |
| | Fig. 1 N1s XPS spectra of Acs. | |
Table 2 showed types of N surface functional groups. In the certain temperature, the activated carbon would reacted with the melamine or urea during the activation process. Therefore pyrrolic N and pyridinic could be observed in XPS. N-5 that could withstand the high activation temperatures accounted for the majority of nitrogen functionalities. AC2 had more pyridinic-N group than that of other two ACs. N-Q was not found in all AC. Scheme 1 showed that the transformation of N functional groups during modification process by urea and melamine. Moreover, the different modifier had almost a little impact on the chemical state of N.
Table 2 Distribution of N species obtained from the deconvolution of the N1s peaks
| Sample |
AC1 |
AC2 |
AC3 |
| N-6, pyridinic nitrogen (398.7 ± 0.3 eV) |
14.9 |
17.9 |
14.2 |
| N-5, pyrollic nitrogen, pyridone (400.3 ± 0.3 eV) |
73.9 |
71.2 |
71.0 |
| N-Q, quaternary nitrogen (401.4 ± 0.4 eV) |
|
|
|
| N-X, oxidized nitrogen (402–405 eV) |
11.2 |
10.9 |
14.8 |
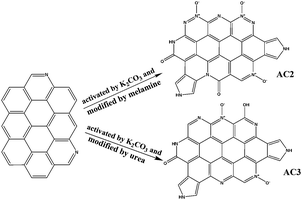 |
| | Scheme 1 The transformation of N functional groups during modification process. | |
Pore structure analysis
The analyses of the N2 isotherms were performed by applying the BET equation, t-plot and DFT methods. Physical properties of the ACs, such as surface areas, total pore volumes, were shown in Table 3, respectively. As can be seen from Table 3, the maximum BET surface and micropore volume of AC3 modified with urea could get 1511 m2 g−1 and 0.688 cm3 g−1, respectively.
Table 3 Physical properties of the Acs
| Sample |
SBET (m2 g−1) |
Smi (m2 g−1) |
Sme (m2 g−1) |
Vtot (cm3 g−1) |
Vme (cm3 g−1) |
Wp (nm) |
| AC1 |
1120 |
1013 |
38 |
0.43 |
0.11 |
1.94 |
| AC2 |
1438 |
1372 |
34 |
0.71 |
0.06 |
1.99 |
| AC3 |
1511 |
1452 |
37 |
0.69 |
0.06 |
1.82 |
Fig. 2 plotted the nitrogen adsorption–desorption isotherms of ACs. The curve trends among all the ACs for volume adsorbed were very similar. The isotherms displayed an abrupt increase in adsorbed volume at a low relative pressure (p/p0). As the relative pressure increased, the nitrogen uptake increased across the entire pressure range. The shape of isotherm corresponded to type I, indicating that the ACs were essentially microporous,14 which can be illustrated from Fig. 3 and Table 3. From Fig. 3, it could be seen that the adsorption capacity of AC modified with urea attained a higher value.
 |
| | Fig. 2 Nitrogen adsorption isotherm by ACs. | |
 |
| | Fig. 3 The curves of differential distribution of pore sizes of ACs. | |
FTIR
Fig. 4 showed the FTIR spectra of Acs. All samples exhibited the OH stretching vibrations band (the one located at the wavenumbers, 3436 cm−1) of surface hydroxylic groups. In addition, the relative increase in bands characteristics of –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N moieties (in the 2925 cm−1) in AC2 and AC3. These suggested the additional modification about N happened in the carbon surface of AC2 and AC3 instead of AC1. Below 2000 cm−1, the FTIR spectra of the carbon samples demonstrated absorption typical of surface and structural oxygen species. In all samples, the wavenumbers of the νC
N moieties (in the 2925 cm−1) in AC2 and AC3. These suggested the additional modification about N happened in the carbon surface of AC2 and AC3 instead of AC1. Below 2000 cm−1, the FTIR spectra of the carbon samples demonstrated absorption typical of surface and structural oxygen species. In all samples, the wavenumbers of the νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibrations (the one located at the wavenumbers, 1632 cm−1) and –C–O–C– (in the 1079 cm−1) could be observed.
C vibrations (the one located at the wavenumbers, 1632 cm−1) and –C–O–C– (in the 1079 cm−1) could be observed.
 |
| | Fig. 4 FTIR spectra of Acs over 400 to 4000 cm−1. | |
Batch equilibrium studies
Effect of contact time. 0.1 g activated carbon samples were put in 250 mL flasks containing 100 mL of aqueous Cr(VI) solution. The initial concentration of Cr(VI) was 100 mg L−1 and the pH was 2. The results are shown in Fig. 5. It can be seen from Fig. 5 that the adsorption of Cr(VI) on the activated carbon increased slowly with time and attains equilibrium. In the beginning, the Cr(VI) adsorbed selectively occupied the active sites on the adsorbent. As contact time increased, the active sites on the adsorbent were filled and adsorption of Cr(VI) became gradually slower and reached a plateau. There was no significant increase in adsorption when contact time increased from 6 to 9 h. Therefore, optimum contact time for all further experiments was set at 9 h. Cr(VI) adsorption capacity of AC2 and AC3 from bamboo residues was 89 mg g−1 and 85 mg g−1, respectively. Cr(VI) removal was as high as 89% and 85%. Chen et al. reported that the Cr(VI) adsorption capacity of AC purchased from Shanghai Chemical Corp was 72.31 mg g−1.15 Cr(VI) adsorption capacity of AC1 without modification was 79 mg g−1.
 |
| | Fig. 5 Effect of contact time on adsorption of Cr(VI) (carbon dosage = 0.1 g; Cr(VI) concentration = 100 mg L−1). | |
Compared with AC2 and AC3, AC2 modified with melamine had the lower surface area and higher N content, but the Cr(VI) adsorption capacity was higher than that of AC3, which mainly due to that the more introduction of N-containing surface groups made activated carbon more alkaline, especially pyridinic N group increased adsorption of Cr(VI).
Effect of solution pH. The effect of solution pH on the Cr(VI) removal was examined by varying the initial pH of the solutions from 1 to 8. The Cr(VI) initial concentration was 100 mg L−1, with an activated lignin dosage of 1 g L−1 and a solution temperature of 20 °C. pH values were adjusted with 0.1 M HCl and 0.1 M NaOH. As can be seen from Fig. 6, Cr(VI) adsorption favored acidic conditions, i.e. pH 2, with a maximum uptake of 72.9%, 88.7%, 84.9% for AC1, AC2 and AC3, respectively. The maximum adsorption of Cr(VI) was explained by higher hydrogen ion (H+) concentration at low pH values. At pH 2, the predominant Cr(VI) was HCrO4−. When the pH is increased above 2, adsorption of Cr(VI) decreased. It was mainly because that increasing the pH will shift the concentration of HCrO4− to other forms (CrO42− and Cr2O72−). So, it can be concluded that the active form of Cr(VI) that can be adsorbed effectively is HCrO4−.
 |
| | Fig. 6 Effect of pH on adsorption of Cr(VI) (carbon dosage = 0.1 g; Cr(VI) concentration = 100 mg L−1; equilibration time = 9 h). | |
Effect of Cr(VI) initial concentration. 100 mL of Cr(VI) solutions with initial concentrations of 50–280 mg L−1 were prepared in a series of flasks to study the effect of Cr(VI) initial concentration on the adsorption uptake. 0.1 g of the activated carbon was added into each flask covered with a glass stopper. Equilibrium experiments were carried out at room temperature (20 °C), and the solution pH was adjusted at 2, and the results are shown in Fig. 7. It was found that the adsorption of Cr(VI) was strongly dependent on initial metal ion concentration. As shown in Fig. 7, the equilibrium adsorption capacities increased when initial concentration of Cr(VI) ions increased. Comparing with adsorption capacities of Cr(VI) onto AC1, AC2 and AC3, the equilibrium adsorption capacities of AC2 were higher under each initial Cr(VI) concentration than that of other two samples. The adsorption capacity of AC1, AC2 and AC3 is 111.8, 133.5, and 125.0 mg g−1, respectively.
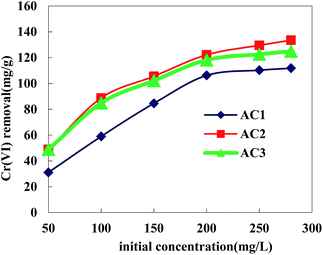 |
| | Fig. 7 Effect of Cr(VI) initial concentration on adsorption (AC dosage = 0.1 g L−1; equilibration time = 9 h; pH = 2). | |
Adsorption kinetic studies. The study of adsorption kinetics is significant as it provides valuable insights into the reaction pathways and the mechanism of the reactions. The effect of contact time on adsorption of Cr(VI) onto studied ACs is shown in Fig. 5. All experimental kinetic data were fitted to the commonly applied pseudo-first-order equation and pseudo-second-order equation.16 These kinetic rate equations can be written as follows:| |
ln(qe − q) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t
| (3) |
| |
 | (4) |
where q (mg g−1) is the amount of Cr(VI) adsorbed at time t (min); qe (mg g−1) is the amount of Cr(VI) adsorbed at equilibrium, kl (1/min) is the first-order adsorption rate const. In Fig. 7 the fitting plots using pseudo-first-order equation and pseudo-second-order equation were shown. The kinetic parameters acquired from fitting results were summarized in Table 4. The data showed that good agreement between the experimental and the calculated qe values was observed by using the pseudo-second-order equation. Obtained results suggested that AC2 and AC3 adsorption system are not first order reaction and that the pseudo-second order model provides the best correlation of the data, the introduction of N-group made ACs more alkaline, with accordance the assumption that the rate-limiting step may be caused by chemical sorption or chemisorption involving valency forces through sharing or exchange of electrons between adsorbent and adsorbate (Fig. 8).
Table 4 Kinetic parameters of different adsorption rate
| Carbon type |
Pseudo-first-order equation |
Pseudo-second-order equation |
| qe (mg g−1) |
k1 (min−1) |
R2 |
qe (mg g−1) |
k2 (g mg min−1) |
R2 |
| AC2 |
88.75 |
0.006 |
0.92 |
100 |
0.00017 |
0.994 |
| AC3 |
84.87 |
0.007 |
0.95 |
100 |
0.00015 |
0.995 |
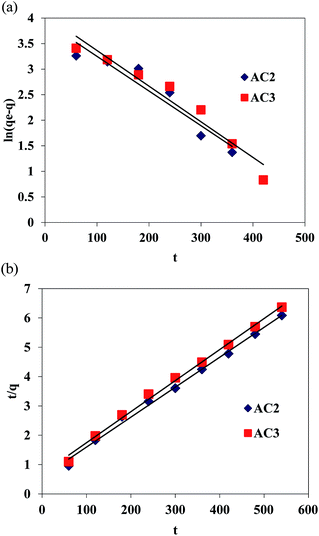 |
| | Fig. 8 (a) The kinetic fitting plots pseudo-first-order equation. (b) The kinetic fitting plots pseudo-second-order equation. | |
In addition, the intraparticle diffusion kinetic model is also been used to analyze the adsorption processes. It can be written as follows:
where
kid is the intraparticle diffusion rate constant (mg g
−1 min
−0.5) and
C is the intercept which represents the thickness of boundary layer effect.
17
Table 5 and Fig. 9 show the intraparticle diffusion plot. Due to the intercept is not zero which indicates surface sorption and intraparticle diffusion are rate controlling processes. The processes of the sorption can be divided into two phases. The first phase is the diffusion controlled by external surface. The second phase has been assigned to intraparticle diffusion.
Table 5 Kinetic parameters of the intraparticle diffusion
| Carbon type |
kid (min−1) |
C |
R2 |
| AC2 |
1.96 |
45.8 |
0.95 |
| AC3 |
2.09 |
38.7 |
0.099 |
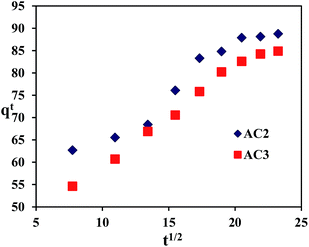 |
| | Fig. 9 The intraparticle diffusion plots for adsorption. | |
Adsorption isotherms. Langmuir adsorption isotherm was given by the following equation| |
 | (6) |
where qm (mg g−1) is the amount of adsorption corresponding to complete monolayer coverage and b (1/mg) is the Langmuir constant related to the energy or net enthalpy of adsorption. The linear plots of Ce/qe versus Ce were shown in Fig. 10.
 |
| | Fig. 10 (a) The linear from Langmuir equation. (b) The linear from Freundlich equation. | |
Freundlich adsorption isotherm was given by the following equation
| |
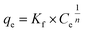 | (7) |
where
Kf and
n are Freundlich constants related to adsorption capacity and adsorption intensity, for a good adsorbent, 1 <
n < 10. The above relation was presented in
Fig. 10, the value of the coefficient of determination (
R2) ranged from 96.7% to 99.7% and was higher for Langmuir isotherm than for the Freundlich isotherm, which ranged from 94.4% to 98.5%. The adsorption isotherm of Cr(
VI) for two AC samples exhibited Langmuir behavior, which indicates that the uptake occurs on a homogenous surface by monolayer adsorption and can be described in terms of chemisorptions as the formation of an ionic or covalent bonds between adsorbent and adsorbate. Since the value of 1/
n was less than 1, it indicated a favorable adsorption (
Table 6).
Table 6 Parameters of different isotherms of adsorption
| Carbon type |
Langmuir |
Freundlich |
| qm (mg g−1) |
b |
R2 |
n |
Kf |
R2 |
| AC2 |
137.0 |
0.14 |
0.995 |
4.95 |
49.9 |
0.985 |
| AC3 |
142.9 |
0.13 |
0.996 |
5.24 |
49.1 |
0.944 |
Conclusions
Utilization of the N-enriched bamboo residue-based ACs for the treatment of aqueous solution containing Cr(VI) ions is a simple, effective and economical method of wastewater treatment. The mechanism of Cr(VI) ion binding to ACs may include ion exchange, surface adsorption, chemisorption and complexation. The sorption capacity is 89 mg g−1 for AC2 with N content of 6.269% modified with melamine, and 85 mg g−1 for AC3 with N content of 4.717% modified with urea. Effective Cr(VI) uptake was favored at low pH values between 2.0 and 4.0. The adsorption of Cr(VI) increased with an increase in the concentrations of these metals in solution. Cr(VI) adsorption on these two N-enriched bamboo residue based ACs was described by the Langmuir isotherm model and the adsorption kinetic followed pseudo-second-order model. And this adsorption was controlled by surface sorption and intraparticle diffusion which could be proved through the intraparticle diffusion kinetic model. These well implied that the activated carbon adsorption was heterogeneous monolayer adsorption and the adsorption of Cr(VI) was spontaneous, favorable and practical. The substantially lower cost, easily available of the bamboo residue indicates great potential for the removal of Cr(VI) ions from aqueous systems.
Acknowledgements
The authors are greatly indebted to the National Natural Science Funding under Project 51172028: study on the preparation of new chemical warfare materials based stem core of han hamp and self-desorption mechanism to the chemical warfare agent and the State Forestry Administration under Project 201204807: the study on the technology and mechanism of the activated carbon electrode from waste hard board.
Notes and references
- G. Arslan and E. Pehlivan, Bioresour. Technol., 2007, 98, 2836–2845 CrossRef CAS PubMed.
- A. B. Albadarin, A. H. Al-Muhtaseb, G. M. Walker, S. J. Allen and M. N. M. Ahmad, Desalination, 2011, 274, 64–73 CrossRef CAS PubMed.
- W. J. Jiang, Q. Cai and W. Xu, Environ. Sci. Technol., 2014, 48, 8078–8085 CrossRef CAS PubMed.
- Y. Wu, S. Z. Zhang, X. Y. Guo and H. L. Huang, Bioresour. Technol., 2008, 99, 7709–7715 CrossRef CAS PubMed.
- X. J. Jin, Z. M. Yu and Y. Wu, Cellul. Chem. Technol., 2012, 46, 79–85 CAS.
- Y. Wang, X. J. Wang, M. Liu, X. Wang, Z. Wu, L. Z. Yang, S. Q. Xia and J. F. Zhao, Ind. Crops Prod., 2012, 39, 81–88 CrossRef CAS PubMed.
- A. Penas-Sanjuan, R. Lopez-Garzon, M. Domingo-Garcia, F. J. Lopez-Garzon, M. Melguizo and M. Perez-Mendoza, Carbon, 2012, 50, 3977–3986 CrossRef CAS PubMed.
- X. J. Jin, M. Y. Zhang, Y. Wu, J. Zhang and J. Mu, Ind. Crops Prod., 2013, 43, 617–612 CrossRef CAS PubMed.
- L. G. Wang, J. Environ. Manage., 2012, 102, 79–87 CrossRef CAS PubMed.
- R. Dobrowolski and M. Otto, Adsorption, 2010, 16, 279–286 CrossRef CAS.
- J. Machnikowski, B. Grzyb, J. V. Weber, E. Frackowiak, J. N. Rouzaud and F. Beguin, Electrochim. Acta, 2004, 49, 423–432 CrossRef CAS PubMed.
- K. Jurewicz, K. Babel, A. Ziolkowski and H. Wachowska, Electrochim. Acta, 2003, 48, 1491–1498 CrossRef CAS.
- Y. J. Kim, Y. Abe, T. Yanaglura, K. C. Park, M. Shimizu, T. Iwazaki, S. Nakagawa, M. Endo and M. S. Dresselhaus, Carbon, 2007, 45, 2116–2125 CrossRef CAS PubMed.
- S. X. Liu, X. Chen, X. Y. Chen, Z. F. Liu and H. L. Wang, J. Hazard. Mater., 2007, 141, 315–319 CrossRef CAS PubMed.
- T. Chen, T. Wang, D. J. Wang, H. R. Xue, J. Q. Zhao, X. C. Ding, S. C. Wu and J. P. He, Mater. Res. Bull., 2011, 46, 1424 CrossRef CAS PubMed.
- Y. S. Ho, J. C. Y. Ng and G. McKay, Sep. Purif. Methods, 2000, 29, 189–232 CrossRef CAS PubMed.
- W. J. Jiang and M. Pelaez, Chem. Eng. J., 2013, 222, 527–533 CrossRef CAS PubMed.
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, the resulting mixture were soaked in a 50% K2CO3 solution for 16 h at the mass ratio impregnation (3
3, the resulting mixture were soaked in a 50% K2CO3 solution for 16 h at the mass ratio impregnation (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The samples were then activated at 800 °C for 60 min in nitrogen atmosphere. The obtained ACs modified with melamine and urea were marked AC2 and AC3, respectively, and the controlled sample activated at 800 °C for 60 min in nitrogen atmosphere with K2CO3 at the mass ratio of (3
1). The samples were then activated at 800 °C for 60 min in nitrogen atmosphere. The obtained ACs modified with melamine and urea were marked AC2 and AC3, respectively, and the controlled sample activated at 800 °C for 60 min in nitrogen atmosphere with K2CO3 at the mass ratio of (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) without modification was marked AC1. After carbonization and activation process, all samples were boiled first with 1 M HCl solution and then washed with distilled water until the pH of solution reach to about 6–7. Finally, these ACs were dried at 105 °C in an oven for 6–8 h.
1) without modification was marked AC1. After carbonization and activation process, all samples were boiled first with 1 M HCl solution and then washed with distilled water until the pH of solution reach to about 6–7. Finally, these ACs were dried at 105 °C in an oven for 6–8 h.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) were ground, and then desorbed at room temperature and pressed to obtain IR-transparent pellets. The measurements were carried out over 4000–400 cm−1.
100) were ground, and then desorbed at room temperature and pressed to obtain IR-transparent pellets. The measurements were carried out over 4000–400 cm−1.

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N moieties (in the 2925 cm−1) in AC2 and AC3. These suggested the additional modification about N happened in the carbon surface of AC2 and AC3 instead of AC1. Below 2000 cm−1, the FTIR spectra of the carbon samples demonstrated absorption typical of surface and structural oxygen species. In all samples, the wavenumbers of the νC
N moieties (in the 2925 cm−1) in AC2 and AC3. These suggested the additional modification about N happened in the carbon surface of AC2 and AC3 instead of AC1. Below 2000 cm−1, the FTIR spectra of the carbon samples demonstrated absorption typical of surface and structural oxygen species. In all samples, the wavenumbers of the νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibrations (the one located at the wavenumbers, 1632 cm−1) and –C–O–C– (in the 1079 cm−1) could be observed.
C vibrations (the one located at the wavenumbers, 1632 cm−1) and –C–O–C– (in the 1079 cm−1) could be observed.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t
qe − k1t