Peroxidase-like activity of ferric ions and their application to cysteine detection†
Xiao-Qiong Wua,
Yan Xua,
Yi-Lin Chenb,
Huan Zhaoa,
Hao-Jie Cuia,
Jiang-Shan Shen*a and
Hong-Wu Zhang*a
aKey Laboratory of Urban Pollutant Conversion, Institute of Urban Environment, Chinese Academy of Sciences, Xiamen, 361021, China. E-mail: jsshen@iue.ac.cn; hwzhang@iue.ac.cn
bCollege of Materials Science and Engineering, Huaqiao University, Xiamen, 361021, China
First published on 19th November 2014
Abstract
Although Fe3O4 magnetic nanoparticles (MNPs) have recently been developed as artificial enzymes in a wide range of applications, there is a debate on whether the observed peroxidase-like activity originates from the nature of the intact MNPs themselves or from the Fenton reaction of surface bound and free ferric/ferrous ions. In this work, Fe3+ ions serving as the peroxidase mimic towards the 3,3′,5,5′-tetramethyl benzidine (TMB)–H2O2 system was investigated in detail for the first time. Experimental results revealed that the peroxidase-like activity of Fe3+ ions is much higher than that of Fe3O4 MNPs. On the basis of these findings, a simple, highly sensitive and selective colorimetric sensing platform for L-cysteine (L-Cys) was developed with a limit of detection (LOD) as low as 0.97 μM.
Introduction
Great efforts have been spent by scientists to find some enzyme mimics with oxidase-, peroxidase- and catalase-like activity to substitute natural enzymes.1–3 The reason is that natural enzymes usually suffer from several disadvantages, for example, (1) they can be easily inactivated in the presence of proteases or denatured under inappropriate experimental conditions such as high temperature and very acidic/basic pH; (2) the preparation, purification and storage of natural enzymes are troublesome.4 Among all kinds of enzyme mimics, peroxidase mimics have been drawn much more attention due to their great potential in practical applications.In 2007, Fe3O4 magnetic nanoparticles (MNPs) called nanoenzymes were firstly reported by Yan's group to demonstrate intrinsic peroxidase-like activity,5 similar to that of natural peroxidases such as horseradish peroxidase (HRP) which containing Fe3+ ions. Although Fenton reaction or Fenton-like reaction has been known for a long time, the observed peroxidase-like activity was suggested by the authors to result from the nature of intact MNPs themselves. Since this pioneering work, a series of nanomaterials with the peroxidase-like activity including FeS,6,7 AgX (X = Cl, Br, I),8 Au,9,10 Ag,11,12 CoFe2O4,13,14 CeO2,15 CuO,16 CuS,17 and Co3O418,19 nanomaterials have been successfully developed as enzyme mimics applied in the fields of chemo-/biosensing and even environmental chemistry. In particular, iron-based nanomaterials such as Fe3O4 MNPs and iron-containing coordination complexes/polymers have been widely explored due to their intriguing peroxidase-like activity.20–23
However, these artificial nanomaterials served as enzyme mimics have several drawbacks, for instance, (1) the procedures of preparing nanomaterials with high quality in general are complicated and difficult; (2) intrinsic instability of artificial nanomaterials can susceptibly cause the surface oxidation/aggregation which lead to the decrease and even loss of their catalytic activity. These shortcomings seriously limit their practical applications, in particular in the field of chemo-/biosensing. Therefore, developing simple and stable enzyme mimics are important to afford much more reliable substitutes possessing the activity of natural enzyme. Furthermore, the observed peroxidase-like activity were in public debated to originate from whether the nature of intact MNPs themselves or the Fenton reaction of surface bound and free ferric/ferrous ions, yet no unanimous conclusion was reached at present.5,24–26 In addition, besides Fe3O4 MNPs and HRP, some coordination complexes/polymers containing Fe3+ or Cu2+ ions have also been investigated to show similar peroxidase-like activity.20,22,27,28 Given growing interests in developing Fe3O4 and iron-based nanomaterials or complexes/polymers as enzyme mimics, probing the peroxidase-like activity of ferric/ferrous ions is thus important for affording much more useful insights for the nature of catalytic activity of these enzyme mimics yet lacking.
Herein, we firstly employed Fe3+ ions as a peroxidase mimic to catalyze the 3,3′,5,5′-tetramethyl benzidine (TMB)–H2O2 system serving as a model redox reaction. The experimental conditions including the effects of pH, temperature, H2O2 and Fe3+ ions concentrations were systemically investigated. The apparent steady-state kinetic parameters for the catalytic reaction were also determined. Importantly, our experiments revealed that Fe3+ ions could exhibit substantially higher peroxidase-like activity than that of Fe3O4 MNPs when Fe concentration was set to exactly the same. Besides Fe3+ ions, Fe2+ and Cu2+ ions could also show similar peroxidase-like activity. On the basis of these findings and in view of the high binding capability of L-Cys towards Fe3+ ions by means of forming Fe-SR coordination binding, the catalytic system was further employed to construct a simple, highly selective and sensitive sensing platform for L-cysteine (L-Cys) which is a SH-containing natural amino acid acting as an important structural and functional component of many proteins and enzymes.29
Results and discussion
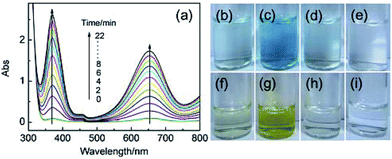
To investigate the peroxidase-like activity of Fe3+ ions, TMB was employed as a model substrate in the presence of H2O2 taking advantage of the good stability of TMB and its product.30 Experimental results showed that Fe3+ ions could effectively catalyze the TMB–H2O2 system which was indicated by the appearance of blue color of resulting solution (Fig. 1c). However, this catalytic reaction did not occur if H2O2 or Fe3+ ions in the system were absent. The time dependent UV-vis absorption spectra of the Fe3+–TMB–H2O2 system were demonstrated as Fig. 1a. Similarly, o-phenylenediamine (OPD) as another peroxidase substrate could also be catalyzed by Fe3+ ions in the presence of H2O2 to produce yellow color. These results revealed that Fe3+ ions possess the peroxidase-like activity similar to those of reported nanomaterials such as Fe3O4 MNPs and iron-containing coordination complexes/polymers.5,20,22,25,27,28 The catalytic activity of Fe3+ ions towards the TMB–H2O2 system was found to be dependent on pH, temperature, both H2O2 and Fe3+ ions concentrations (Fig. S1–S4, see the ESI†). The pH and temperature were about 4.0 and 50 °C respectively to obtaining optimal catalytic efficiency (Fig. S1 and S2†). It should be pointed out that Fe3+ ions required a H2O2 concentration of 4 mM to reach the maximal level of peroxidase activity and the peroxidase activity could be slightly suppressed when H2O2 concentration was further increased (Fig. S3†), similar to those of reported other peroxidase mimics and HPR.5,21,22 The effect of Fe3+ ions concentration on the catalytic rate indicated that higher concentration of Fe3+ ions could result in faster catalytic reaction. Importantly, even if Fe3+ ions concentration was decreased to 0.2 μM which was 3 orders of magnitude lower than that of TMB fixed at 0.5 mM, a significant catalytic reaction could still be occurred (Fig. S4†). Unambiguously, this observation was indicative of the peroxidase-like activity of Fe3+ ions. Fe2+ and Cu2+ ions were also found to show similar peroxidase-like activity towards the TMB–H2O2 system, and further experiments revealed that the peroxidase-like activity of Fe2+ ions is almost as same as that of Fe3+ ions (Fig. S5†).The apparent steady-state kinetic parameters for the reaction were measured by the initial rate method. The absorbance at 652 nm wavelength was employed to afford the corresponding concentration of the oxidized TMB product by using the molar absorption coefficient of 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1.31 Within the suitable range of TMB (Fig. 2a) and H2O2 (Fig. 2b) concentrations, the typical Michaelis–Menten curves were observed. The data were well fitted to the Michaelis–Menten model to obtain the catalytic parameters given in Table S1.† It should be noted that the apparent Michaelis–Menten constant Km with TMB as the substrate is much higher than that with H2O2 as the substrate. This indicated that Fe3+ ions showed a much higher affinity towards H2O2 than that towards TMB, different from most of other reported nanomaterials as peroxidase mimics.5 The parallel lines obtained from double-reciprocal plots (Fig. S6†) further revealed that a ping-pong mechanism should be responsible for the reaction of the TMB–H2O2 system catalyzed by Fe3+ ions, which could make a conclusion that Fe3+ ions can bind and react with the first substrate and the first product is released before reacting with the second substrate.32
000 M−1 cm−1.31 Within the suitable range of TMB (Fig. 2a) and H2O2 (Fig. 2b) concentrations, the typical Michaelis–Menten curves were observed. The data were well fitted to the Michaelis–Menten model to obtain the catalytic parameters given in Table S1.† It should be noted that the apparent Michaelis–Menten constant Km with TMB as the substrate is much higher than that with H2O2 as the substrate. This indicated that Fe3+ ions showed a much higher affinity towards H2O2 than that towards TMB, different from most of other reported nanomaterials as peroxidase mimics.5 The parallel lines obtained from double-reciprocal plots (Fig. S6†) further revealed that a ping-pong mechanism should be responsible for the reaction of the TMB–H2O2 system catalyzed by Fe3+ ions, which could make a conclusion that Fe3+ ions can bind and react with the first substrate and the first product is released before reacting with the second substrate.32
However, several studies claimed that the peroxidase-like activity of Fe3O4 MNPs should originate from the nature of the intact nanomaterials themselves rather than the leached ferric/ferrous ions5,6,25,26 for the leached Fe concentration in solution could only be determined to ∼21.2 μg L−1 (equal to ∼0.4 μM Fe) which considered to be lower than the concentration required for the Fenton reaction.5 Furthermore, the amount of leached ferric/ferrous ions from Fe3O4 MNPs is strongly pH-dependent.24,25 However, this is considerable contradictory with our present observation on the effect of Fe3+ ions concentration, because even 0.2 μM Fe3+ ions could still induce significant catalytic reaction towards the TMB–H2O2 system (Fig. S4†). To afford much more useful insights for this problem, two kinds of Fe3O4 MNPs with different average sizes, ∼6 nm and ∼400 nm (Fig. S7†) were employed to conduct as control experiments. The results revealed that when the total Fe concentration of the Fe3O4 MNPs of size 400 nm was fixed at 5 μM measured by atomic absorption spectrophotometry the MNPs did not catalyze the TMB–H2O2 system (Fig. 3a) which was quite different from the Fe3+ ions case. However, increasing the total Fe concentration in the MNPs case, the catalytic reaction could be gradually facilitated. Similar experimental results were observed in the case of Fe3O4 MNPs of size 6 nm (Fig. 3b). It should be pointed out that for 400 nm MNPs, even the total Fe concentration was enhanced up to 1000 μM, 200 times higher than that of the Fe3+ ions case, poor catalytic performance was still observed, however, when the total Fe concentration of 6 nm MNPs was 20 times higher than that of Fe3+ ions case, the catalytic rates in both MNPs and Fe3+ ions cases were almost the same (Fig. 3). Therefore, it could conclude that the catalytic activity of Fe3O4 MNPs with different sizes was in the order of 6 nm > 400 nm. These observations also indicated that the peroxidase-like activity of Fe3+ ions was much higher than that of Fe3O4 MNPs.
The deficiency of L-Cys is considered to be involved in several diseases.33 Therefore, developing rapid, simple, sensitive and selective detection strategies for L-Cys are highly demanded. On the basis of our findings, a high selective and sensitive sensing platform for L-Cys could be further developed. Considering the strong complexation capability of thiols towards Fe3+ ions, the catalytic reaction of the Fe3+–TMB–H2O2 system can be inhibited by adding L-Cys. As shown in Fig. 4, the catalytic reaction was gradually suppressed with increasing L-Cys concentration and good linear relationship was found between [L-Cys] and the natural logarithm of absorbance at 652 nm wavelength when 15 min of reaction time was taken. The linear correlation of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A = 0.168–8278.25 [L-Cys] (R2 = 0.997) was obtained over the tested concentration range of L-Cys from 0 to 50 μM when 5 μM Fe3+ ions was employed (Fig. 4a and d). The limit of detection (LOD) for L-Cys was estimated to be 6 μM (3σ/k, n = 11). However, when the concentration of Fe3+ ions was further reduced to 2 μM and 0.8 μM, similar linear correlations between ln
A = 0.168–8278.25 [L-Cys] (R2 = 0.997) was obtained over the tested concentration range of L-Cys from 0 to 50 μM when 5 μM Fe3+ ions was employed (Fig. 4a and d). The limit of detection (LOD) for L-Cys was estimated to be 6 μM (3σ/k, n = 11). However, when the concentration of Fe3+ ions was further reduced to 2 μM and 0.8 μM, similar linear correlations between ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A and [L-Cys] were still observed with a LOD lowered to 1.5 μM and 0.97 μM, respectively (Fig. 4b, c, e and f). Obviously, the LOD could be lowered with decreasing Fe3+ ions concentration. It could thus conclude that a signalling amplification was shown in this catalytic system. These LODs are better than those of some reported chemosensors which are 10 μM and 21.3 μM.34,35 To further support that the inhibition of the catalytic reaction originated from the formation of Fe-SR, inorganic S2− was employed to replace L-Cys in this catalytic system and similar experimental results were observed (Fig. S8†). The inhibition of the catalytic reaction was thus ascribed to forming Fe-SR compounds due to high stability constant of Fe-SR which could result in reducing the amount of unbound Fe3+ ions. It should be noted that, the color of the Fe3+–TMB–H2O2–Cys system was found to gradually return to blue when the reaction time was extended to more than 20 min which may be resulting from the dissociation of Fe-SR compounds due to the oxidation of excess H2O2.6,7,36 Other 19 natural amino acids were employed to test the selectivity of this catalytic system. The results showed that under similar experimental conditions employing other amino acids with concentration of 15-fold higher than that of L-Cys did not suppress the catalytic reaction which was indicative of good selectivity (Fig. 5). As shown in Fig. 5, Tyr, Trp, Lys and His somewhat promote this reaction. The observed positively catalytic effect likely resulted from their certain oxidize activity of these amino acids which is obviously different from other tested amino acids. Although numerous colorimetric or fluorometric systems for sensing L-Cys have been developed,37–44 to the best of our knowledge, an approach for sensing L-Cys based on the peroxidase-like activity of Fe3+ ions or Fe3O4 MNPs has not been reported so far.
A and [L-Cys] were still observed with a LOD lowered to 1.5 μM and 0.97 μM, respectively (Fig. 4b, c, e and f). Obviously, the LOD could be lowered with decreasing Fe3+ ions concentration. It could thus conclude that a signalling amplification was shown in this catalytic system. These LODs are better than those of some reported chemosensors which are 10 μM and 21.3 μM.34,35 To further support that the inhibition of the catalytic reaction originated from the formation of Fe-SR, inorganic S2− was employed to replace L-Cys in this catalytic system and similar experimental results were observed (Fig. S8†). The inhibition of the catalytic reaction was thus ascribed to forming Fe-SR compounds due to high stability constant of Fe-SR which could result in reducing the amount of unbound Fe3+ ions. It should be noted that, the color of the Fe3+–TMB–H2O2–Cys system was found to gradually return to blue when the reaction time was extended to more than 20 min which may be resulting from the dissociation of Fe-SR compounds due to the oxidation of excess H2O2.6,7,36 Other 19 natural amino acids were employed to test the selectivity of this catalytic system. The results showed that under similar experimental conditions employing other amino acids with concentration of 15-fold higher than that of L-Cys did not suppress the catalytic reaction which was indicative of good selectivity (Fig. 5). As shown in Fig. 5, Tyr, Trp, Lys and His somewhat promote this reaction. The observed positively catalytic effect likely resulted from their certain oxidize activity of these amino acids which is obviously different from other tested amino acids. Although numerous colorimetric or fluorometric systems for sensing L-Cys have been developed,37–44 to the best of our knowledge, an approach for sensing L-Cys based on the peroxidase-like activity of Fe3+ ions or Fe3O4 MNPs has not been reported so far.
Conclusions
In summary, we showed for the first time the peroxidase-like activity of Fe3+ ions that could catalyse some peroxidase substrates including TMB and OPD to form coloured products. The steady-state kinetic investigation revealed that the catalytic behavior of Fe3+ ions towards the TMB–H2O2 system as a model redox reaction was well fitted to the Michaelis–Menten model. Our experiments also indicated that Fe3+ ions could exhibit much higher peroxidase-like activity than that of Fe3O4 MNPs. On the basis of our findings, a simple, stable, highly selective and sensitive sensing platform was thus developed for L-Cys. Several intriguing enlightenments could be summarized in the present system, for example, (1) the present system is much more simple and stable compared with the cases of artificial Fe3O4 MNPs for the reason that the preparation procedures of MNPs are laborious and MNPs are intrinsic instable during storage and preparation; (2) it not only can afford much more useful insights for the nature of the observed peroxidase-like activity of Fe3O4 MNPs which considered to originate from the overall results of Fenton reaction of surface bound and leached ferric/ferrous ions rather than the nature of the intact MNPs themselves, but also can show some hints for the nature of reported other metal or metal oxides/sulfides nanomaterials including CuO, CuS, CeO2, and FeS with peroxidase-like or oxidase-like activity; (3) employing catalytic reaction could construct a highly selective and sensitive sensing system for L-Cys featured by a signalling amplification which was different from most of previous reported spectral sensing systems for Cys just on the basis of a specific reaction between L-Cys and a chromophore or fluorophore; (4) considering the excellent coordination capability of Fe3+ ions, Fe3+-containing coordination complexes or polymers bearing specifically recognizing sites for biological targets such as cancer cells are expected to be easily designed and prepared in the future.45Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant no. 21377124), by Natural Science Foundation of Ningbo (Grant no. 2013A610034), by Science and Technology Project in Xiamen (Grant no. 3502Z20132012), and by National Key Technology Support Program (Grant no. 2012BAC25B04).Notes and references
- R. Breslow, Acc. Chem. Res., 1995, 28, 146 CrossRef CAS.
- G. Wulff, Chem. Rev., 2002, 102, 1 CrossRef CAS PubMed.
- J.-M. Zen and A. S. Kumar, Acc. Chem. Res., 2001, 34, 772 CrossRef CAS PubMed.
- D. L. Nelson and M. M. Cox, Lehninger Principles of Biochemistry, W. H. Freeman & Co Ltd., New York, 4th edn, 2005, ch. 6 Search PubMed.
- L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett and X. Yan, Nat. Nanotechnol., 2007, 2, 577 CrossRef CAS PubMed.
- Z. Dai, S. Liu, J. Bao and H. Ju, Chem.–Eur. J., 2009, 15, 4321 CrossRef CAS PubMed.
- A. K. Dutta, S. K. Maji, D. N. Srivastava, A. Mondal, P. Biswas, P. Paul and B. Adhikary, ACS Appl. Mater. Interfaces, 2012, 4, 1919 CAS.
- G. L. Wang, X. F. Xu, L. Qiu, Y. M. Dong, Z. J. Li and C. Zhang, ACS Appl. Mater. Interfaces, 2014, 6, 6434 CAS.
- Y. Jv, B. Li and R. Cao, Chem. Commun., 2010, 46, 8017 RSC.
- Y. J. Long, Y. F. Li, Y. Liu, J. J. Zheng, J. Tang and C. Z. Huang, Chem. Commun., 2011, 47, 11939 RSC.
- K. S. McKeating, S. Sloan-Dennison, D. Graham and K. Faulds, Analyst, 2013, 138, 6347 RSC.
- H. Jiang, Z. Chen, H. Cao and Y. Huang, Analyst, 2012, 137, 5560 RSC.
- W. Shi, X. Zhang, S. He and Y. Huang, Chem. Commun., 2011, 47, 10785 RSC.
- Y. Fan and Y. Huang, Analyst, 2012, 137, 1225 RSC.
- Q. Wang, Z. Yang, X. Zhang, X. Xiao, C. K. Chang and B. Xu, Angew. Chem., Int. Ed., 2007, 46, 4285 CrossRef CAS PubMed.
- W. Chen, J. Chen, A. L. Liu, L. M. Wang, G. W. Li and X. H. Lin, ChemCatChem, 2011, 3, 1151 CrossRef CAS.
- W. He, H. Jia, X. Li, Y. Lei, J. Li, H. Zhao, L. Mi, L. Zhang and Z. Zheng, Nanoscale, 2012, 4, 3501 RSC.
- J. Mu, Y. Wang, M. Zhao and L. Zhang, Chem. Commun., 2012, 48, 2540 RSC.
- W. Qin, L. Su, C. Yang, Y. Ma, H. Zhang and X. J. Chen, Agric. Food Chem., 2014, 62, 5827 CrossRef CAS PubMed.
- L. Ai, L. Li, C. Zhang, J. Fu and J. Jiang, Chem.–Eur. J., 2013, 19, 15105 CrossRef CAS PubMed.
- Y. Chen, H. Cao, W. Shi, H. Liu and Y. Huang, Chem. Commun., 2013, 49, 5013 RSC.
- J. Tian, S. Liu, Y. Luo and X. Sun, Catal. Sci. Technol., 2012, 2, 432 CAS.
- W. Wang, X. Jiang and K. Chen, Chem. Commun., 2012, 48, 7289 RSC.
- C. Yang, J. Du, Q. Peng, R. Qiao, W. Chen, C. Xu, Z. Shuai and M. Gao, J. Phys. Chem. B, 2009, 113, 5052 CrossRef CAS PubMed.
- H. Wei and E. Wang, Anal. Chem., 2008, 80, 2250 CrossRef CAS PubMed.
- R. Cui, Z. Han and J. J. Zhu, Chem.–Eur. J., 2011, 17, 9377 CrossRef CAS PubMed.
- A. Singh, S. Patra, J.-A. Lee, K. H. Park and H. Yang, Biosens. Bioelectron., 2011, 26, 4798 CrossRef CAS PubMed.
- C. Hsiao, I.-C. Chou, C. D. Okafor, J. C. Bowman, E. B. O'Neill, S. S. Athavale, A. S. Petrov, N. V. Hud, R. M. Wartell, S. C. Harvey and L. D. Williams, Nat. Chem., 2013, 5, 525 CrossRef CAS PubMed.
- Z. A. Wood, E. Schröder, J. R. Harris and L. B. Poole, Trends Biochem. Sci., 2003, 28, 32 CrossRef CAS.
- X. Zuo, C. Peng, Q. Huang, S. Song, L. Wang, D. Li and C. Fan, Nano Res., 2009, 2, 617 CrossRef CAS.
- E. I. Karaseva, Y. P. Losev and D. I. Metelitsa, Russ. J. Bioorg. Chem., 2002, 28, 128 CrossRef CAS.
- S. Y. Chen, P. Chen, Y. W. Chen and J. G. Wang, Catalytic Reaction Kinetics, Chemical Industry Press, 2007 Search PubMed.
- S. Shahrokhian, Anal. Chem., 2001, 73, 5972 CrossRef CAS.
- M. Zhang, Z.-B. Qu, C.-M. Han, L.-F. Lu, Y.-Y. Li, T. Zhou and G. Shi, Chem. Commun., 2014, 50, 12855–12858 RSC.
- D. P. Murale, H. Kim, W. S. Choi and D. G. Churchill, Org. Lett., 2013, 15, 3630 CrossRef CAS PubMed.
- S. K. Maji, A. K. Dutta, P. Biswas, D. N. Srivastava, P. Paul, A. Mondal and B. Adhikary, Appl. Catal., A, 2012, 170, 419–420 Search PubMed.
- X. Chen, Y. Zhou, X. Peng and J. Yoon, Chem. Soc. Rev., 2010, 39, 2120 RSC.
- Y. Guo, S. Shao, J. Xu, Y. Shi and S. Jiang, Tetrahedron Lett., 2004, 45, 6477 CrossRef CAS PubMed.
- Q. Qian, J. Deng, D. Wang, L. Yang, P. Yu and L. Mao, Anal. Chem., 2012, 84, 9579 CrossRef CAS PubMed.
- R. P. Briñas, M. Hu, L. Qian, E. S. Lymar and J. F. Hainfeld, J. Am. Chem. Soc., 2008, 130, 975 CrossRef PubMed.
- O. Rusin, N. N. S. Luce, R. A. Agbaria, J. O. Escobedo, S. Jiang, I. M. Warner, F. B. Dawan, K. Lian and R. M. Strongin, J. Am. Chem. Soc., 2004, 126, 438 CrossRef CAS PubMed.
- D. Zhang, M. Zhang, Z. Liu, M. Yu, F. Li, T. Yi and C. Huang, Tetrahedron Lett., 2006, 47, 7093 CrossRef CAS PubMed.
- J.-S. Shen, D.-H. Li, M.-B. Zhang, J. Zhou, H. Zhang and Y.-B. Jiang, Langmuir, 2011, 27, 481 CrossRef CAS PubMed.
- Y. B. Ruan, A. F. Li, J. S. Zhao, J. S. Shen and Y. B. Jiang, Chem. Commun., 2010, 46, 4938 RSC.
- A. Asati, S. Santra, C. Kaittanis, S. Nath and J. M. Perez, Angew. Chem., Int. Ed., 2009, 48, 2308 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S8 and Table S1. See DOI: 10.1039/c4ra11000e |
| This journal is © The Royal Society of Chemistry 2014 |