Stable superhydrophobic surface based on silicone combustion product
Lie Shen*a,
Wenlian Qiua,
Bin Liua and
Qipeng Guob
aMOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou 310027, China. E-mail: shenlie@zju.edu.cn; Fax: +86-571-87953712; Tel: +86-571-87953712
bPolymers Research Group, Institute for Frontier Materials, Deakin University, Locked Bag 2000, Geelong, Victoria 3220, Australia. E-mail: qipeng.guo@deakin.edu.au
First published on 14th October 2014
Abstract
Discarded silicone products can be recycled to prepare superhydrophobic powder by simply burning and smashing. The powder can be used to fabricate a superhydrophobic surface with mechanical durability such that the superhydrophobicity was kept after 50 abrasion cycles. A robust electroconductive superhydrophobic surface can also be obtained by this simple method.
Superhydrophobic surfaces with a water contact angle (WCA) above 150° and a sliding angle (SA) below 10° are appealing to both scientific research and engineering applications1–4 for their wide applicability in self-cleaning, corrosion-resistant, water–oil separation and organic solar cells.5–8 In addition to the initial mimicking of a lotus leaf, multiple biomimetic materials have been fabricated inspired by other natural species for their excellent properties, including nacre9,10 and cocoon silk11. Up to now, many methods of preparing artificial superhydrophobic surfaces have been developed.12–15 However, not only did most of the methods demand special equipment and expensive materials, but the created superhydrophobic surfaces also lacked mechanical durability, which hindered their use in practical applications.14,16–20 Harsh conditions, such as water flow impact and mechanical touch, could destroy the micro-scale rough structure which is crucial for superhydrophobicity.13,14,18,21–23 Fabricating superhydrophobic surfaces with mechanical durability has become an urgent demand for every effort. If self-superhydrophobic materials are obtained and applied to prepare superhydrophobic surfaces, which to some degree are like lotus leaves retaining non-wettability by regenerating an epicuticular wax layer after being damaged, the mechanical stability problem will be mitigated.
Silicone elastomers based on polydimethylsiloxane (PDMS) are widely applied in the fields of high-voltage outdoor insulation, encapsulation and seal rings. Recently, they have been used to fabricate superhydrophobic surfaces24–26 due to PDMS's multiple properties, such as low toxicity, long-term stability and a wide range of operating temperatures. PDMS (Sylard 184) was successfully used to prepare a transparent superhydrophobic coating mixed with silica nanoparticles.27 Otherwise, silicone elastomer products are widely used in daily life, for instance, silicone cups, silicone phone shells and silicone toys. With increasing amounts of silicone elastomer products being consumed, how to sufficiently reuse discarded silicone elastomer products is an important aspect.
Herein, we report a kind of superhydrophobic powder with microstructures which can be obtained by simply burning silicone and then pulverizing. By a single step of hot-pressing, the powder can be utilized to prepare a superhydrophobic powder/polymer composite surface with a high water contact angle (WCA) and low sliding angle (SA), possessing excellent mechanical durability that can retain superhydrophobicity after 50 cycles of abrasion tests. The consequent excellent stable superhydrophobicity is likely to provide a new approach to reusing discarded silicone products to fabricate non-wetting surfaces for practical applications. The combustion product of silicone containing 16 wt% Ketjen black was pulverized into powder and hot-pressed on the polymer surface, and a stable electroconductive superhydrophobic surface can be fabricated.
Sylard-184A and Sylard-184B (RTV-2) were mixed in a mass ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
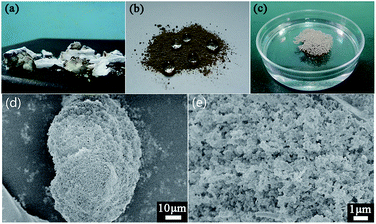
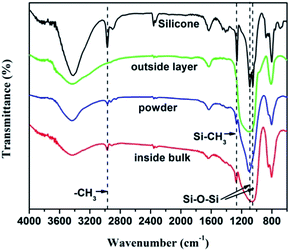
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and cured at ambient temperature. Cured silicone was cut into small pieces and held in the flame of an alcohol lamp in air until the silicone pieces were completely burned. As shown in Fig. 1a, the silicone combustion product is composed of two parts: a white layer outside and khaki bulk inside. FT-IR spectra (Fig. 2) display a series of absorption bands ranging from 800 to 3600 cm−1, implying the presence of Si–O–Si bonds, Si–CH3 groups and methyl groups.28,29 Compared to silicone, the absorption intensities of Si–CH3 groups and methyl groups decrease remarkably in the inside bulk and are inexistent in the outside layer, suggesting that various degrees of degradation occurred inside and outside of the silicone piece when burning. The silicone outer was completely combusted, attributable to direct contact with oxygen, whereas the inside part degraded into short chains of polysiloxane due to being oxygen-deficient. The pulverized silicone combustion product (powder for short) is a mixture of the outside layer and inside bulk.
1 and cured at ambient temperature. Cured silicone was cut into small pieces and held in the flame of an alcohol lamp in air until the silicone pieces were completely burned. As shown in Fig. 1a, the silicone combustion product is composed of two parts: a white layer outside and khaki bulk inside. FT-IR spectra (Fig. 2) display a series of absorption bands ranging from 800 to 3600 cm−1, implying the presence of Si–O–Si bonds, Si–CH3 groups and methyl groups.28,29 Compared to silicone, the absorption intensities of Si–CH3 groups and methyl groups decrease remarkably in the inside bulk and are inexistent in the outside layer, suggesting that various degrees of degradation occurred inside and outside of the silicone piece when burning. The silicone outer was completely combusted, attributable to direct contact with oxygen, whereas the inside part degraded into short chains of polysiloxane due to being oxygen-deficient. The pulverized silicone combustion product (powder for short) is a mixture of the outside layer and inside bulk.
 | ||
| Fig. 2 FT-IR spectra of curved silicone, the outside layer, inside bulk after silicone piece combustion and pulverized powder of the silicone combustion product. | ||
Notably, it was found that the powder possessed superhydrophobicity when sprinkled randomly on a flat surface as shown in Fig. 1b; water droplets exhibited spherical shapes on the powder surface. When sprinkled in water, the powder floated on water without any wetting and the reflection in water could be seen clearly (Fig. 1c). Even after a month, the power was completely dry and still floated on water. Fig. 1d and e show the morphologies of the powder. It can be seen that the powder surface is uniformly and densely covered with pores, very coarse just like a honeycomb; at a higher magnification it reveals that the powder consists of densely packed nanospheres and pores, with large numbers of microscale protrusions like coral reefs and pores distributed on it, forming a loose micro- and nanoscale rough structure. We believe that it is the hierarchical coralloid structure of the powder that leads to the feature of superhydrophobicity. It may be an interesting discovery to obtain such a kind of superhydrophobic silicon material through simply combusting silicone. Meaningfully, we collected some discarded silicone products and burned them completely; the resulting combustion product possessed the same property of superhydrophobicity as the RTV-2.
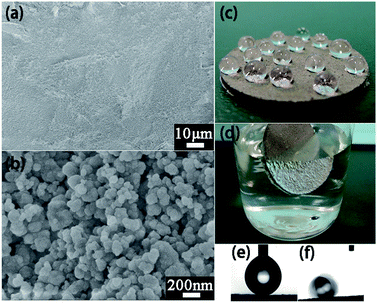
Two factors are essential for preparing superhydrophobic surfaces: low surface energy materials and micro and nanoscale roughness. Inspired by the micro and nanoscale rough morphology and pressure endurance, the powder was utilized to fabricate a superhydrophobic surface. Referring to the previous one-step hot-press method,30 powder was distributed on the bottom of a disc mold, then put in a polypropylene (PP) disc piece and the mold was pressed under a pressure of 10 MPa for 30 min at 180 °C. As shown in Fig. 3a, the composite surface is flat, just like the morphology of the densely packed powder; at a higher magnification we can see that the nano-particles with diameter of 100–200 nm stack together (Fig. 3b), forming a robust rough structure which remained after being smashed and pressed. The water repellency of the coating is highlighted in Fig. 3c; the water droplets exhibit spherical shapes on the powder/PP composite surface. Upon immersion in water, the surface acts like a silver mirror when viewed at a glancing angle (Fig. 3d), and the surface was completely dry after being removed from the water without any mass change before and after immersion. The bright, reflective surface visible in the water reveals that an air layer exists between water and the superhydrophobic surface.31 The porous rough morphology of the surface traps air and thus establishes surface solid–liquid–air interfaces,32 leading to excellent superhydrophobicity with a WCA of 160° (Fig. 3e) and a SA of 3.5° (Fig. 3f).
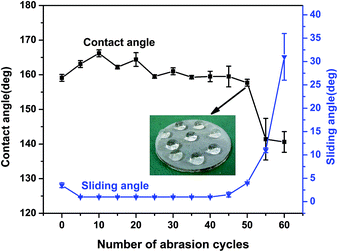
In practical applications, the mechanical resistance of the superhydrophobic surface is crucial and it needs to survive harsh conditions. To investigate the mechanical stability of the composite surface, sandpaper abrasion tests were performed. Sandpaper (600 mesh) was used as an abrasion surface, with the tested superhydrophobic surface facing it. The superhydrophobic surface was dragged in one direction at a speed of 3 cm s−1, and an abrasion length of 28 cm, corresponding to a pressure of 5.7 kPa. The powder was not sufficiently robust to completely resist sandpaper abrasion. The amount of removed powder embedded on the surface increased with the increasing abrasion test cycles. Owing to the powder's self-superhydrophobicity and the surface's self-similarity, the surface did not lose its superhydrophobicity until the powder layer was completely removed. Fig. 4 shows that within 50 abrasion cycles, the composite surface retained superhydrophobicity and the WCA was kept around 160° while the SA was below 5°. Repeated abrasion did not cause a loss in the surface's superhydrophobicity until the powder surface disappeared, only leaving a pure polymer substrate surface. The composite surface lost superhydrophobicity after being abraded for 55 abrasion cycles. The WCA decreased from 160° to 140° and the SA changed from 3.5° to 31° after 60 abrasion cycles. The results indicate that the powder/polymer composite surface possessed excellent superhydrophobicity durability and abrasion resistance that can be applied in practical use.
Superhydrophobic surface functionalizations are required in many practical applications, such as transparency and electroconductivity. We found that an electroconductive superhydrophobic surface can be obtained through the same method as mentioned above. 16 wt% Ketjen-black (KB300) was added into Sylgard-184A and stirred homogeneously, which was then mixed with Sylgard-B at a mass ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The cured silicone was burned completely and smashed. The powder was hot-pressed on the PP surface under a pressure of 10 MPa for 30 min at 180 °C, and a conductive superhydrophobic surface was obtained. We used a RTS-4 four-probe conductivity meter to investigate the surface's sheet resistance and found it to be 103 Ω sq−1. The same sandpaper abrasion tests were also performed on the electroconductive superhydrophobic surface. After 30 abrasion cycles, the surface still retained superhydrophobicity with the WCA of 162°, SA of 4° and electroconductivity with sheet resistances of 103 Ω sq−1. The obtained conductive superhydrophobic surface with mechanical stability simultaneously has potential uses as static charge resistance and electromagnetism interference shielding materials. Moreover, the powder can be also prepared by recycling and combusting discarded electroconductive silicone products, opening an avenue for reusing them.
1. The cured silicone was burned completely and smashed. The powder was hot-pressed on the PP surface under a pressure of 10 MPa for 30 min at 180 °C, and a conductive superhydrophobic surface was obtained. We used a RTS-4 four-probe conductivity meter to investigate the surface's sheet resistance and found it to be 103 Ω sq−1. The same sandpaper abrasion tests were also performed on the electroconductive superhydrophobic surface. After 30 abrasion cycles, the surface still retained superhydrophobicity with the WCA of 162°, SA of 4° and electroconductivity with sheet resistances of 103 Ω sq−1. The obtained conductive superhydrophobic surface with mechanical stability simultaneously has potential uses as static charge resistance and electromagnetism interference shielding materials. Moreover, the powder can be also prepared by recycling and combusting discarded electroconductive silicone products, opening an avenue for reusing them.
Conclusions
In summary, an easy-to-obtain water-repellent silica material was made from silicone elastomer combustion. Utilizing the powder, a superhydrophobic powder/polymer surface can be fabricated by a simple hot-pressing step. The composite surface possessed excellent mechanical durability that can retain superhydrophobicity after 50 cycles of an abrasion test. An electroconductive superhydrophobic surface can also be obtained through the same method by burning silicone containing an electroconductive filler or electroconductive silicone products. The electroconductive superhydrophobic surface kept its superhydrophobicity and electroconductivity after 30 abrasion cycles. This study has provided a new approach to reuse discarded silicone products to fabricate non-wetting surfaces. It is expected that the non-wetting silicone combustion product can be applied in more fields for practical use.Acknowledgements
We gratefully acknowledge that the project was supported by Zhejiang Provincial Natural Science Foundation of China (no. LY13E030001).Notes and references
- C. Neinhuis and W. Barthlott, Ann. Bot., 1997, 667 CrossRef.
- H. Y. Erbil, Y. Avcl and O. Mert, Science, 2003, 299, 1377 CrossRef CAS PubMed.
- X. Gao, X. Yan, X. Yao, L. Xu, K. Zhang, J. Zhang, B. Yang and L. Jiang, Adv. Mater., 2007, 19, 2213 CrossRef CAS.
- L. Shen, H. L. Ding, Q. H. Cao, W. C. Jia, W. Wang and Q. P. Guo, Carbon, 2012, 50, 4284 CrossRef CAS PubMed.
- J. Genzer and K. Efimenko, Biofouling, 2006, 22, 339 CrossRef CAS PubMed.
- A. Tuteja, W. Chio, M. L. Ma, J. M. Mabry, S. A. Mazzella, G. C. Rutledge, G. H. McKinley and R. E. Cohen, Science, 2007, 318, 1618 CrossRef CAS PubMed.
- Q. Zhu, Y. Chu, Z. K. Wang, N. Chen, L. Lin, F. T. Liu and Q. M. Pan, J. Mater. Chem. A, 2013, 1, 5386 CAS.
- X. Deng, L. Mammen, Y. F. Zhao, P. Lellig, K. Müllen, C. Li, H. J. Butt and D. Vollmer, Adv. Mater., 2011, 23, 2962 CrossRef CAS PubMed.
- Q. Cheng, L. Jiang and Z. Tang, Acc. Chem. Res., 2014, 47, 1256 CrossRef CAS PubMed.
- J. Wang, Q. Cheng and Z. Tang, Chem. Soc. Rev., 2012, 41, 1111 RSC.
- M. Wu, H. Shuai, Q. Cheng and L. Jiang, Angew. Chem., Int. Ed., 2014, 53, 3358 CrossRef CAS PubMed.
- E. Celia, T. Darmanin, E. T. Givenchy, S. Amigoni and F. Guittard, J. Colloid Interface Sci., 2013, 402, 1 CrossRef CAS PubMed.
- X. Zhu, Z. Zhang, X. Men, J. Yang, K. Wang, X. Xu, X. Zhao and Q. Xue, J. Mater. Chem., 2011, 21, 15793 RSC.
- X. Deng, L. Mammen, H. J. Butt and D. Vollmer, Science, 2012, 335, 67 CrossRef CAS PubMed.
- Z. X. Zhang, Y. Li, M. Ye, K. Boonkerd, Z. Xin, D. Vollmer, J. K. Kim and X. Deng, J. Mater. Chem. A, 2014, 2, 1268 CAS.
- X. Zhang, F. Shi, J. Niu, Y. G. Jiang and Z. Q. Wang, J. Mater. Chem., 2008, 18, 621 RSC.
- X. M. Li, D. Reinhoudt and M. Grego-Calama, Chem. Soc. Rev., 2007, 36, 1350 RSC.
- T. Verho, C. Bower, P. Andrew, S. Franssila, O. Ikkala and R. H. A. Ras, Adv. Mater., 2010, 19, 2213 Search PubMed.
- M. Callies and D. Quere, Soft Matter, 2005, 1, 55 RSC.
- J. S. Lee, J. C. Yoon and J. H. Jang, J. Mater. Chem. A, 2013, 1, 7312 CAS.
- P. Roach, N. J. Shirtcliffe and M. I. Newton, Soft Matter, 2008, 4, 224 RSC.
- X. Y. Ling, I. Y. Phang, G. J. Vancso, J. Huskens and D. N. Reinhoudt, Langmuir, 2009, 25, 3260 CrossRef CAS PubMed.
- E. Huovinen, L. Takkunen, T. Korpela, M. Suvanto, T. T. Pakkanen and T. A. Pakkanen, Langmuir, 2014, 30, 1435 CrossRef CAS PubMed.
- F. Wang, S. Yu, J. Ou, M. Xue and W. Li, J. Appl. Phys., 2013, 114, 124902 CrossRef PubMed.
- L. Shen, W. Wang, H. L. Ding and Q. P. Guo, Appl. Surf. Sci., 2013, 284, 651 CrossRef CAS PubMed.
- Z. Shen, C. Hou, S. Liu and Z. Guan, J. Appl. Polym. Sci., 2014, 10, 1002 Search PubMed.
- Z. K. He, M. Ma, X. R. Lan, F. Chen, K. Wang, H. Deng, Q. Zhang and Q. Fu, Soft Matter, 2011, 7, 6435 RSC.
- S. S. Latthe, H. Imai, V. Ganesan and A. V. Rao, Microporous Mesoporous Mater., 2010, 130, 115 CrossRef CAS PubMed.
- A. V. Rao, A. B. Gurav, S. S. Latthe, R. S. Vhatkar, H. Imai, C. Kappenstein, P. B. Wagh and S. C. Gupta, J. Colloid Interface Sci., 2010, 352, 30 CrossRef CAS PubMed.
- M. Peng, Z. J. Liao, J. Qi and Z. Zhou, Langmuir, 2010, 26, 13572 CrossRef CAS PubMed.
- I. A. Larmour, S. E. J. Bell and G. C. Saunders, Angew. Chem. Int., Ed., 2007, 46, 1710 CrossRef CAS PubMed.
- A. B. D. Cassie and S. Baxter, Trans. Faraday Soc., 1944, 40, 546 RSC.
| This journal is © The Royal Society of Chemistry 2014 |