Fluorescent metal–organic framework based on pyrene chromophore for sensing of nitrobenzene†
Shuilin Xiea,
Haifeng Wanga,
Zhonghua Liub,
Renke Daia and
Lizhen Huang*a
aSchool of Bioscience and Biotechnology, South China University of Technology, Guangzhou, 510006, China. E-mail: huanglzh@scut.edu.cn; Tel: +86-13631406992
bNeurology Department, Zhongshan City People's Hospital, Zhongshan, 528403, China
First published on 8th December 2014
Abstract
A highly luminescent metal–organic framework (MOF) constructed by a rare dendritic multicarboxyl acid with a pyrene chromophore was synthesized successfully. The fluorescence intensity of the MOF in DMF is obviously stronger than that in other common solvents such as acetone, CH2Cl2, CHCl3, cyclohexane, toluene, pyridine and THF. This particular fluorescence response to DMF is significant in sensing DMF. In addition, this MOF can sense nitrobenzene effectively. Low detection limit and good reproducibility make this material promising for explosive substance detection.
Introduction
In the past few decades, metal–organic frameworks (MOFs) have gained the attention of many crystal engineers from all over the world because of their delicate architectures,1 topological diversity,2 controllable channels,3 and a great many potential applications such as gas storage and separation,4 molecule sensing and recognizing,5 catalysis,6 optical and magnetism,7 bioimaging8 and so on. The tunable size and porosity of these materials are in favor of chemical sensing because high internal surface areas and large cavities can concentrate analytes to levels high above those in the surrounding atmosphere and can lead to much smaller detection levels. A fast and sensitive detection of explosive constituents is of great importance for environmental monitoring. Fluorescence quenching based sensing is a common strategy for its simplicity and sensitivity.9 Till now, many reports about MOFs as sensors for nitroaromatic explosive sensing, which confirmed their potential application in sensor fields. However, designing and synthesizing a luminescent MOF with fast and sensitive fluorescence response is still challenging.To get a luminescent MOF, a common method is to design and synthesize a fluorescent organic ligand. For this purpose, many aromatic rigid molecules such as benzene, naphthalene, anthracene, carbazole, triphenylamine were used for ligands synthesizing. However, ligands with a pyrene chromophore are very few because of their synthesizing complexities and poor solubilities. In the year of 2010, Rosseinsky et al. reported a InMOF constructed with the first pyrene based ligand, tetrakis(p-benzoic acid)pyrene (TBAPy).10 After that, a few MOFs based on this ligand had been reported by Rosseinsky and Hupp.11 Recently, Zhu et al. reported a new MOF constructed by another pyrene based ligand, 1,3,6,8-tetrakis(3,5-isophthalic acid)pyrene (H8TIAPy).12 All these pyrene based MOFs showed fascinating fluorescent properties.
Herein, we report another MgMOF (1) constructed with the ligand H8L (Scheme 1). The crystals of 1 are strongly fluorescent when dispersed in DMF and show sensitive response to nitrobenzene through a fluorescence quenching mechanism. In addition, good repeatability and small detection level make this material promising for sensing application.
Experimental section
Chemicals and materials
All solvents and reagents were bought from commercial sources as analytical grade and directly used without further purification. The ligand was synthesized according to a reported literature.12 Powder X-ray diffraction (PXRD) measurements were performed on a Rigaku DMAX 2550 diffractometer at 50 kV, 20 mA for Cu Kα (λ = 1.5418 Å). Thermogravimetric analysis (TGA) was performed on a Perkin-Elmer thermogravimetric analyzer with a heating rate of 5 °C per minute. Fluorescence measurement was conducted on FLUOROMAX-4 spectrometer.Results and discussion
Structure and characterization
The ligand H8L was synthesized according to a reported literature. The reaction of the ligand with Mg(NO3)2 in DMF at 85 °C for 2 days gave the yellow block crystals of 1 formulated as Mg4(L)(DMF)4(H2O)4(DMF)0.5 confirmed by single crystal X-ray diffraction and TG analysis with a good yield. Single crystal X-ray analysis reveals that 1 crystallized in the monoclinic space group P21/c with the lattice parameters a = 11.064 Å, b = 15.957 Å, c = 23.362 Å, β = 92.265° which was isomorphic with JUC-118 reported by N. Zhao et al.12 However, the coordinating style and the terminal are different from JUC-118. Each ligand connects to eight Mg2 clusters and each Mg2 cluster connects to four ligands (Fig. 1a). The two Mg atoms in the cluster are both six-coordinated. Mg1 is coordinated by three carboxyl oxygen atoms from the ligands, two oxygen atoms from terminal disordered DMF molecules and one oxygen atom from water which is also splitted. Mg2 is coordinated by five carboxyl oxygen atoms from the ligands among which one takes the μ2-O style and one oxygen atom from water (Fig. 1b). In addition, the conformation of the ligand was different from JUC-118. The torsion angels between the pyrene and the benzene were 42.59 and 49.78 degrees in 1 of this work and 44.59 and 52.88 degrees in JUC-118 and 54.69 and 60.78 degrees in JUC-118-1 (Fig. S6†).12 The assemble of the clusters and ligands give a three dimensional (3D) porous framework (Fig. 1c). The solvent accessible volume was 62.0% calculated by platon. Attempt for getting the BET surface was failed because of the rapid collapse of the framework upon activation in vacuum. The topology analysis of the framework was conducted by the TOPOS 4.0 program.13 Each benzene ring in the ligand could be simplified as a three-connected node and the pyrene as a four-connected node and each Mg2 cluster as a four-connected one. Thus, the whole framework of 1 could be simplified as a 3,3,4,4-net (Fig. 1d), the point symbol for net was {4 6 8}2{4 62 83}2{62 82 10 12}{62 8}2.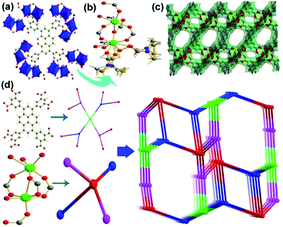 | ||
| Fig. 1 (a) The coordination style of the ligand. (b) The dinuclear Mg2 cluster. (c) The 3D structure of 1. (d) Topology simplification strategy. | ||
The similarity between the simulated and experimental powder X-ray diffraction (PXRD) confirmed the phase purity (Fig. S5†). TG analysis showed that 1 started to decompose around 400 °C and the weight loss before 350 °C could be mainly attributed to the isolated guest molecules in the channels and coordinated solvent molecules (Fig. S2†).
Fluorescence property and sensing of organic solvent molecules
As the pyrene is a strong fluorescent chromophore, the ligand and 1 are both luminescent. The maximum emission of the ligand is at about 540 nm, while the maximum emission of 1 is at about 490 nm (Fig. S3†). The blue fluorescence shift of 1 relative to the ligand might be caused by metal-to-ligand charge transfer (MLCT). In order to research the fluorescence of 1 further, we had soaked the same amount crystals of 1 in different common solvents (DMF, acetone, CH2Cl2, CHCl3, cyclohexane, toluene, pyridine and THF), treated with ultrasonication, and then aged to form stable emulsions prior to fluorescence measurements. As shown in Fig. 2, the fluorescence intensity of 1 in DMF was obvious stronger which was almost twice than that in other solvents. The particular fluorescence response to DMF was of significant interest in the sensing of DMF, which is very harmful to human health.Sensing of nitrobenzene
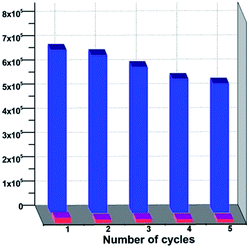
Nitrobenzene is a common solvent in organic synthesis, but it is extremely poisonous to human beings. 1 was highly luminescent and can easily sense nitrobenzene by a fluorescence quenching mechanism. 5 mg crystals of 1 were added to 3 ml DMF and sonificated for about 10 minutes to get a good disperse. And then different amounts of nitrobenzene were added to this suspension. The fluorescence intensities were recorded, respectively. As shown in Fig. 3, when the concentration of the nitrobenzene was 80 ppm, the fluorescence began to have an obvious quenching, and when the concentration of nitrobenzene was about 800 ppm, the fluorescence was almost quenched completely. According to the Stern–Volmer equation: I0/I = 1 + Ksv[M], among which [M] means the concentration of the quenching agent, we got the Stern–Volmer curve of 1 and the I0/I had an approximate linear relationship with the nitrobenzene. In addition, this MOF could be regenerated and reused for a significant number of cycles by centrifuging the dispersed solution after nitrobenzene detection and washing several times with fresh DMF. It is noteworthy that almost regaining the initial fluorescence intensity and high quenching efficiency over repeated cycles implied a high photostability of this material (Fig. 4). The low detection level and good repeatability of the MOF made it promising for nitrobenzene detection application. | ||
| Fig. 3 The normalized fluorescence intensities of 1 in DMF with different concentrations of nitrobenzene (left) and the Stern–Volmer curve (right). | ||
Conclusions
A strong fluorescent MgMOF has been synthesized constructed by a rare dendritic multicarboxylic ligand with a pyrene chromophore. The dispersed solution of 1 in DMF exhibits much stronger fluorescence emission in DMF than in other solvents. In addition, the fluorescence of 1 could be effectively quenched by nitrobenzene with a low concentration. Low detection level and good repeatability make this material very promising for explosives detection.Acknowledgements
This work is supported by the Fundamental Research Funds for the Central Universities (2014ZM0067) and National Natural Science Foundation of China (81202585).Notes and references
- (a) M. O'Keeffe, M. A. Peskov, S. J. Ramsden and O. M. Yaghi, Acc. Chem. Res., 2008, 41, 1782–1789 CrossRef PubMed; (b) L. Peng, J. L. Zhang, Z. M. Xue, B. X. Han, X. X. Sang, C. C. Liu and G. Y. Yang, Nat. Commun., 2014, 5, 1678 Search PubMed; (c) Q. L. Zhu and Q. Xu, Chem. Soc. Rev., 2014, 43, 5468–5512 RSC.
- (a) P. J. Ma, Y. H. Li, L. Qin and G. H. Cui, Bull. Korean Chem. Soc., 2013, 34, 3843–3846 CrossRef CAS; (b) Z. J. Xiahou, Y. L. Wang, Q. Y. Liu, J. J. Wei and L. L. Chen, Inorg. Chem. Commun., 2013, 38, 62–64 CrossRef CAS PubMed.
- (a) Y. Cheng, H. Kajiro, H. Noguchi, A. Kondo, T. Ohba, Y. Hattori, K. Kaneko and H. Kanoh, Langmuir, 2011, 27, 6905–6909 CrossRef CAS PubMed; (b) Q. Chen, Z. Chang, W. C. Song, H. Song, H. B. Song, T. L. Hu and X. H. Bu, Angew. Chem., 2013, 52, 11550–11553 CrossRef CAS PubMed.
- (a) L. J. Murray, M. Dinca and J. R. Long, Chem. Soc. Rev., 2009, 38, 1294–1314 RSC; (b) Z. Zhao, X. Ma, A. Kasik, Z. Li and Y. S. Lin, Ind. Eng. Chem. Res., 2013, 52, 1102–1108 CrossRef CAS; (c) S. Van der Perre, T. Van Assche, B. Bozbiyik, J. Lannoeye, D. E. De Vos, G. V. Baron and J. F. M. Denayer, Langmuir, 2014, 30, 8416–8424 CrossRef CAS PubMed.
- (a) Y. Yu, X. M. Zhang, J. P. Ma, Q. K. Liu, P. Wang and Y. B. Dong, Chem. Commun., 2014, 50, 1444–1446 RSC; (b) X. M. Lin, G. M. Gao, L. Y. Zheng, Y. W. Chi and G. N. Chen, Anal. Chem., 2014, 86, 1223–1228 CrossRef CAS PubMed; (c) W. Zhu, C. Wang, W. N. Li, C. A. Tao, J. C. Cui, H. W. Yang, Y. Jiang and G. T. Li, J. Mater. Chem. A, 2013, 1, 11741–11747 RSC; (d) J. H. Wang, M. Li and D. Li, Chem. Sci., 2013, 4, 1793–1801 RSC; (e) D. X. Ma, B. Y. Li, X. J. Zhou, Q. Zhou, K. Liu, G. Zeng, G. H. Li, Z. Shi and S. H. Feng, Chem. Commun., 2013, 49, 8964–8966 RSC; (f) Y. Li, S. S. Zhang and D. T. Song, Angew. Chem., Int. Ed., 2013, 52, 710–713 CrossRef CAS PubMed.
- (a) J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC; (b) K. K. Tanabe and S. M. Cohen, Inorg. Chem., 2010, 49, 6766–6774 CrossRef CAS PubMed.
- (a) M. D. Allendorf, C. A. Bauer, R. K. Bhakta and R. J. Houk, Chem. Soc. Rev., 2009, 38, 1330–1352 RSC; (b) M. Kurmoo, Chem. Soc. Rev., 2009, 38, 1353–1379 RSC.
- W. Morris, W. E. Briley, E. Auyeung, M. D. Cabezas and C. A. Mirkin, J. Am. Chem. Soc., 2014, 136, 7261–7264 CrossRef CAS PubMed.
- (a) B. Gole, A. K. Bar and P. S. Mukherjee, Chem. Commun., 2011, 47, 12137–12139 RSC; (b) Y. C. He, H. M. Zhang, Y. Y. Liu, Q. Y. Zhai, Q. T. Shen, S. Y. Song and J. F. Ma, Cryst. Growth Des., 2014, 14, 3174–3178 CrossRef CAS.
- K. C. Stylianou, R. Heck, S. Y. Chong, J. Bacsa, J. T. A. Jones, Y. Z. Khimyak, D. Bradshaw and M. J. Rosseinsky, J. Am. Chem. Soc., 2010, 132, 4119–4130 CrossRef CAS PubMed.
- (a) K. C. Stylianou, J. Rabone, S. Y. Chong, R. Heck, J. Armstrong, P. V. Wiper, K. E. Jelfs, S. Zlatogorsky, J. Bacsa, A. G. McLennan, C. P. Ireland, Y. Z. Khimyak, K. M. Thomas, D. Bradshaw and M. J. Rosseinsky, J. Am. Chem. Soc., 2012, 134, 20466–20478 CrossRef CAS PubMed; (b) J. E. Mondloch, W. Bury, D. Fairen-Jimenez, S. Kwon, E. J. DeMarco, M. H. Weston, A. A. Sarjeant, S. T. Nguyen, P. C. Stair, R. Q. Snurr, O. K. Farha and J. T. Hupp, J. Am. Chem. Soc., 2013, 135, 10294–10297 CrossRef CAS PubMed; (c) P. Deria, J. E. Mondloch, E. Tylianakis, P. Ghosh, W. Bury, R. Q. Snurr, J. T. Hupp and O. K. Farha, J. Am. Chem. Soc., 2013, 135, 16801–16804 CrossRef CAS PubMed; (d) C. W. Kung, T. C. Wang, J. E. Mondloch, D. Fairen-Jimenez, D. M. Gardner, W. Bury, J. M. Klingsporn, J. C. Barnes, R. Van Duyne, J. F. Stoddart, M. R. Wasielewski, O. K. Farha and J. T. Hupp, Chem. Mater., 2013, 25, 5012–5017 CrossRef CAS; (e) P. Deria, W. Bury, J. T. Hupp and O. K. Farha, Chem. Commun., 2014, 50, 1965–1968 RSC.
- N. Zhao, F. Sun, H. He, J. Jia and G. Zhu, Cryst. Growth Des., 2014, 14, 1738–1743 CAS.
- (a) V. A. Blatov, Struct. Chem., 2012, 23, 955–963 CrossRef CAS PubMed; (b) E. V. Alexandrov, V. A. Blatov, A. V. Kochetkov and D. M. Proserpio, CrystEngComm, 2011, 13, 3947–3958 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Supplementary figures, TG, fluorescence spectrum, UV adsorption spectrum. CCDC 1023832. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra10835c |
| This journal is © The Royal Society of Chemistry 2015 |