Recent developments in Ritter reaction
Dehong Jiang
b,
Tao He
a,
Li Ma
*b and
Zhouyu Wang
*ab
aDepartment of Chemistry, Xihua University, Chengdu 610039, China
bDepartment of Pharmaceutical Engineering, Xihua University, Chengdu 610039, China. E-mail: zhouyuwang77@gmail.com; ma-li@vip.sina.com; Fax: +86-28-87720552
First published on 13th November 2014
Abstract
Ritter reaction is an atom economy reaction which produces an amide through the reaction of a nitrile with an alcohol or alkene in the presence of an acid. A number of important advances have been achieved in recent years with respect to substrates, the variety of catalysts, the reaction media and the diversity of products. This paper reviews recent findings and assesses the Ritter reaction.
1. Introduction
The classical Ritter reaction is the reaction of nitriles with alcohols or alkenes to produce amides in the presence of stoichiometric amounts of sulfuric acid.1 The reaction was first introduced by Ritter in 1948. It has attracted much attention as a result of its atom economy and its applications in the synthesis of biological molecules, especially the preparation of bulky amides.2 However, the large amount of toxic and corrosive acids used in the reaction limits its application. In the last 20 years, Brønsted and Lewis acids have been explored as catalysts in the Ritter reaction. Remarkable progress has been made and developments in the catalytic Ritter reaction were highlighted in 2012.3Recent efforts have been directed toward the development of substrate diversity, the reaction media, new catalyst systems and Ritter-type reaction. Many useful compounds have been synthesized through Ritter or Ritter-type reaction, including asymmetrical di- and tri-substituted ureas, 3-substituted-3-amino-oxindoles, 4-acyl-aminotetrahydroindazoles, N-(4-iodo-1,3-diarylbutyl) acetamides, 4-amidopiperidine derivatives and aza-bicyclic alkaloids.4–8,10,11,13–17,19–24,27,30–36 This review focuses on new findings in Ritter or Ritter-type reaction in the last four years and provides a concise overview of recent progress in this area.
2. Classical Ritter reaction
2.1 Ritter reaction of alcohols
In 2011, Ibrahim et al.4 reported a catalytic Ritter reaction of alcohols with the trimesitylphosphane gold(I) complex [(Mes3P)AuCl, 5 mol%] and AgNTf2 (5 mol%) as a new catalyst system (Scheme 1). This was the first time that gold(I) had been used as a catalyst in the Ritter reaction. Under mild reaction conditions, benzohydric alcohols were transformed to the corresponding amides with moderate to good yields. This method is a valuable alternative to previously published reactions as a result of the mild and neutral conditions required. However, this protocol is not suitable for solid nitriles because nitrile is used as the solvent. In addition, the alcohols are limited to benzohydric alcohols; other secondary alcohols, such as cinnamyl alcohol, 1-ethynylcyclohexanol and di(cylohexyl)-carbinol, resulted in no products.In the same year, pentafluorophenylammonium triflate (PFPAT, 4) was first used as an organocatalyst in a Ritter reaction by Khaksar et al.5 This is an inexpensive, environmentally friendly, simple and efficient method for the synthesis of amides via the Ritter reaction of alcohols with nitriles. Using a catalytic amount of PFPAT (10 mol%), various N-substituted amides were obtained with good to excellent yields (90–95%) in solvent-free conditions at 90 °C after 1–3 h (Scheme 2). Cinammyl alcohol, in particular, is a good substrate for the protocol, whereas normal alcohols are poor substrates for most catalytic Ritter reaction systems. The high reaction temperature required is the main disadvantage of this procedure.
In 2012, we reported an efficient and practical Ritter reaction in subcritical water.6 Trifluoromethanesulfonic acid (TfOH, 20 mol%) was used as the catalyst and sodium dodecyl sulfate (10 mol%) was used as an additive (Scheme 3). The corresponding amides were obtained at high yields. As no organic solvent is used, this procedure is environmentally friendly, non-toxic and inexpensive. This simple catalytic protocol provides a valuable alternative for the preparation of amides. However, the protocol is carried out at high temperatures and is not suitable for normal alcohols.
Silica-bonded N-propyl sulfamic acid (SBNPSA) (0.1 g) was used as an efficient catalyst to produce the corresponding amides at high yields under solvent-free conditions by Shakeri et al.7 (Scheme 4). The solid SBNPSA catalyst has a good ability to accelerate these reactions and all the reactions were completed in short time periods (15–180 min). The catalyst can be recycled and re-used four times without any loss of activity. The protocol is a convenient, mild and efficient method. The only disadvantage of the protocol is the high reaction temperature.
A similar study was reported in 2013 by Ziarani et al.8 They used silica functionalized with sulfonic acid (SiO2-Pr-SO3H, 6) instead of SBNPSA as an environmentally benign, recyclable and highly efficient heterogeneous solid acid catalyst in a Ritter reaction. A series of amides were produced with various nitriles (5.0 mmol) and various tertiary, allylic and benzylic alcohols (5.0 mmol) under solvent-free conditions in the presence of SiO2-Pr-SO3H (0.1 g) at room temperature (Scheme 5). However, the reaction time is prolonged compared with the method of Shakeri et al.7
Ionic liquids have attracted much attention and have been used in extraction and separation, as catalysts and in materials fabrication.9 In 2010, Jiang et al.10 reported the application of Brønsted acid ionic liquids as dual solvent–catalysts for Ritter reaction. A series of various Brønsted acid ionic liquids were synthesized and these were screened as solvent–catalysts for the Ritter reaction. The ionic liquid [NSPTEA][OTF] (7, 3 equiv.) was singled out as the most efficient catalyst for the Ritter reaction at 75 °C under solvent-free conditions (Scheme 6). Under the optimum conditions, various nitriles and tertiary alcohols as well as secondary alcohols were smoothly converted to the corresponding amides in good to excellent yields. Moreover, the ionic liquid [NSPTEA][OTF] was easily separated from the reaction mixtures by extraction with a small amount of water and could be recycled five times without any significant loss of activity. A high catalyst loading (300 mol%) is the main shortcoming in this protocol.
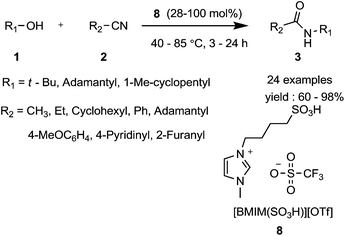
In 2011, Kalkhambkar et al.11 used a Brønsted acid imidazolium ionic liquid [BMIM-(SO3H)][OTf] 8 in the Ritter reaction as a recyclable and convenient catalyst (Scheme 7). Using t-BuOH as the carbo-cation source, a variety of cyclic, acyclic, aromatic and heteroaromatic nitriles reacted under solvent-free conditions to give the corresponding amides in high yields. When adamantanol and methylcyclopentanol were used as carbo-cation precursors, an acid solvent was essential otherwise a poor yield was obtained. This study complements and extends the work of Jiang et al.10
In recent years, microwave technology has developed into a useful technique in synthetic chemistry as a result of fast reaction rates and high yields.12 In 2014, Yaragorla et al.13 introduced the technique into the Ritter reaction (Scheme 8). With Ca(OTf)2 (5 mol%) as a catalyst and Bu4-NPF6 (5 mol%) as an additive, a series of tertiary, secondary and benzyl alcohols reacted with various nitriles to produce the corresponding amides under microwave irradiation. After only 15 min irradiation, good to excellent yields were obtained. However, the reaction is carried out at high temperatures.
Magnetite-supported catalysts have recently emerged as alternatives to solid-supported heterogeneous catalysts as a result of their inert, inexpensive, separable and sustainable characteristics. In 2013, Gawande et al.14 used Nanocat-Fe-OSO3H 9 as a magnetically retrievable sustainable catalyst for the Ritter reaction (Scheme 9). With this promising nano-catalyst, different alcohols and nitriles reacted well with each other under solvent-free conditions. In addition, the catalyst could be used in five reaction cycles without any significant loss of catalytic activity. However, again, a high reaction temperature was necessary.
In 2012, Basavaprabhu and Sureshbabu15 described an application of the classical Ritter reaction. A series of asymmetrical di- and tri-substituted ureas were synthesized at high yields through the reaction of cyanamide and alcohol, catalyzed by an environmentally friendly and safe reagent system (FeCl3, 30 mol%) in dichloromethane. Benzyl and allyl alcohols are good substrates for this protocol. Acetic acid is necessary when the alcohol is tert-butyl alcohol, otherwise a poor yield is obtained (Scheme 10). In view of the mild reaction conditions, the easy availability of the precursors, the atom efficiency and the molecular diversity, this protocol is of significant interest. This was the first time that cyanamide had been used as a substrate in the Ritter reaction. However, as with the ionic liquid catalysts, a high catalyst loading was required.
The 3-substituted-3-amino-oxindoles are very useful and are present in several pharmaceutical candidates. The Ritter reaction of 3-substituted 3-hydroxyoxindoles with nitriles was first used to synthesize these compounds by Zhou et al.16 A series of 3-substituted-3-amino-oxindoles were synthesized from readily available 3-aryl- and 3-alkyl-3-hydroxyoxindoles in CH3CN (0.1 M) or DCE (0.2 M). Perchloric acid (HClO4, 10–20 mol%) was used as the catalyst in the reaction (Scheme 11). This approach adds an efficient method to the toolbox of 3-substituted-3-amino-oxindole chemistry and facilitates the structural modification of the scaffold. A high reaction temperature and long reaction time were required.
In 2012, Turks et al.17 reported a convenient synthetic route toward 4-acylamino-4,5,6,7-tetrahydroindazoles, which are versatile building blocks in medicinal chemistry. The synthetic approach includes the reduction reaction of tetrahydroindazolones to the corresponding 4-hydroxy-tetrahydroindazoles followed by the Ritter reaction with various nitriles. Sulfuric acid (H2SO4, 10 equiv.) in acetic acid (5 mL) solution was used as a strongly ionizing medium (Scheme 12). Various nitriles were used as the substrate and the corresponding 4-acylamino- and 4-amino-substituted tetrahydroindazoles were obtained in good yields. In particular, trichloroacetonitrile is a good substrate for the protocol as it is relatively unreactive compared with other nitriles. The protocol is potentially useful in view of the metal-free procedure, the broad substrate scope and its operational simplicity. The use of corrosive acids as the reaction medium is the main shortcoming of this approach.
Diastereoselective amide formation through the Ritter reaction is a valuable and difficult subject for investigation. A Ritter reaction catalyzed by a diastero-selective acid was first reported by Rubenbauer and Bach in 2009.18 In this study, an asymmetric Ritter reaction was described for chiral secondary benzylic alcohols. Al-Huniti and Lepore19 reported the stereo-retentive Ritter reaction of secondary cycloalkanols. After a systematic evaluation of various metal catalysts, copper(II) triflate [Cu(OTf)2, 20 mol%] was singled out as the best catalyst for the stereo-retentive Ritter reaction of cycloalkanols (Scheme 13). Using this protocol, the corresponding amide products were formed with a near-complete retention of configuration in moderate yields under mild and often solvent-free conditions. The high degree of stereo-retention in this reaction argues against a classical SN1 mechanism. The authors thought that the cyclic carbo-cations in this reaction retained their configuration as a result of stabilization by hyper-conjugation. In this protocol, the copper(II) catalyst chelates both the chlorosulfite and the nitrile coupling partner. This chelation to chlorosulfite increases its leaving group ability, leading to rapid cation formation under mild conditions. This configurationally “frozen” carbo-cation is then trapped by nitrile and finally produces the stereo-retentive amides. The protocol has a general substrate for the nitriles and cycloalkanols and even tolerates a phenolic hydroxyl group. However, the yield of the amide is moderate and the protocol is not suitable for linear alcohols.
2.2 Ritter reaction of alkenes
Although a variety of acid catalysts has been explored for the Ritter reaction of alcohols, only a few catalysts are known for the Ritter reaction of alkenes. In 2010, Reddy et al.20 reported a convenient, simple and efficient method for the synthesis of secondary amides by the Ritter reaction of alkenes. In this protocol, tetrafluoroboric acid etherate (HBF4OEt2, 100 mol%) was first used as a catalyst for the amidation of alkenes with nitriles (Scheme 14). The reaction went to completion at room temperature in 6 h and the corresponding amides were isolated in high yields. Various vinyl arenes, such as p-chloro-, p-methyl- and p-tert-butyl styrenes, underwent smooth coupling with nitriles. The protocol is also effective for sterically hindered 2-vinyl naphthalene, dihydronaphthalene, indene, cyclohexene and cyclopentene. The main limitation of the protocol is the high catalyst loading.In 2012, Y. Hanzawa et al.21 reported another Ritter reaction of alkenes with molecular iodine (I2, 20 mol%) as the catalyst. In their protocol, the reaction of chiral (+)-camphene with benzonitrile under solvent-free conditions in the presence of water (100 mol%) caused skeletal rearrangement of the camphene and amidation, resulting in racemic (±)-N-isobornylbenzamide (19) in good yield (Scheme 15). Under the optimum conditions, the reaction of chiral (+)-camphene with several other aliphatic and aromatic nitriles can occur, producing the corresponding amide compounds in good yields. However, the reactions of several terpenic alkenes (17f, 17g, 17h) and styrene 17i with benzonitrile are not successful. A complex mixture was usually obtained, or no product was produced.
3. Ritter-type reaction
3.1 Ritter-type reaction of esters
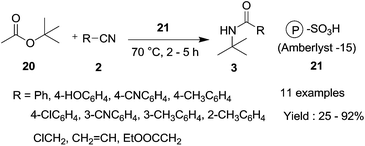
In addition to the progress in classical Ritter reaction, Ritter-type reaction have also been developed in recent years. In 2011, Khaksar et al.5 used PFPAT as an organocatalyst in the classical Ritter reaction of alcohols. Good to excellent yields of different amides were obtained. The protocol was also suitable for the Ritter-type reaction of tert-butyl acetate (Scheme 16). Benzonitrile, acetonitrile, acrylonitrile and 2-phenylacetonitrile all reacted well with tert-butyl acetate in the presence of PFPAT.In 2012, Mokhtary and Goodarzi22 reported a simple, highly efficient methodology for the Ritter-type reaction of tert-butyl acetate. Amberlyst-15 (P-SO3H) was used as an effective and recyclable heterogeneous catalyst in the protocol. The corresponding N-tert-butyl amides were obtained in high yields (Scheme 17). Amberlyst-15 can not only be recovered from the reaction system, but can also be re-used many times without significant loss of catalytic ability. As for the PFPAT catalyst system, a high temperature is required.
In 2012, the same group described another Ritter-type reaction of tert-butyl acetate with polyvinylpolypyrrolidone boron trifluoride (PVPP-BF3, 100 mol%) as the catalyst.23 PVPP-BF3 exhibited high activity in the amidation of tert-butyl acetate with nitriles in 1,2-dichloromethane at 70 °C. A variety of tert-butyl amides was obtained in high yields (Scheme 18). As for the Amberlyst-15 catalyst, PVPP-BF3 can be re-used several times and is easily regenerated. It is thought that the reaction is initiated by the Lewis acidity and oxophilicity of PVPP-BF3, which promotes the Ritter reaction via coordination to the oxygen atoms of the ester and the facilitation of C–O cleavage by the attack of the nitrile group (Scheme 19). The high catalyst loading is the main restriction.
3.2 Ritter-type reaction of ethers
Diethers are often found as intermediates in Ritter reaction. In 2013, Panduranga et al.24 described a simple and mild protocol for the synthesis of N,N-disubstituted ureas from the reaction of cyanamides 10 and readily available ethers. In the presence of boron trifluoride etherate (BF3Et2O, 30 mol%) in acetic acid, various dibenzyl ethers reacted with cyanamides and the corresponding N,N-disubstituted ureas were obtained in good to excellent yields (Scheme 20). The methyl tert-butyl ether and divinyl ether can be used as the ether components to give tert-butyl and allyl ureas, respectively. The corrosive acid used as the reaction medium and the high catalyst loading are the main problems in this protocol.3.3 Ritter-type reaction of alkanes
The direct conversion of C–H bonds to C–N bonds is of particular importance in the synthesis of alkaloids and heterocyclic molecules25 and Ritter-type reaction of alkanes are one method of achieving these syntheses. The first Ritter-type reaction of alkanes was introduced by Olah and Gupta in 1980.26 They reported that adamantane reacts readily with a variety of nitriles in the presence of nitrosonium hexafluorophosphate (NOPF6) to give the corresponding adamantyl amide in high yield at room temperature. In 2011, the [BMIM][PF6]/NOPF6 system was also used to synthesize adamantyl amides from nitriles and adamantane by Kalkhambkar et al. (Scheme 21).11 To obtain a high yield, a high catalyst loading is necessary.In 2012, Michaudel et al.27 reported a new method for the Ritter-type C–H amination of unactivated sp3 carbons in alcohols and ketones. In the protocol, the commercial copper salt CuBr2 (25 mol%), the Lewis acid Zn(OTf)2 (50 mol%) and F-TEDA-PF6 (200 mol%) were used as the catalytic system (Scheme 22). A broad range of saturated alcohols and ketones were used and C–H amination of the unactivated sp3 carbons proceeded. The corresponding γ-amidoalcohols and β-amidoketones were obtained with moderate to good isolated yields. In particular, the protocol allows for the direct, innate C–H amination of different alkanes without the need for pre-functionalization or the installation of a directing group. The transformation of adamantine, cyclopentane, cyclohexane, cycloheptane and cyclo-octane proceeded at room temperature in good yields. This is a promising method that can be used to directly functionalize natural product derivatives. However, the catalyst loading is still high.
3.4 Ritter-type reaction of halohydrocarbons
The first Ritter-type reaction of halohydrocarbons was introduced by Olah et al.28 in 1979. They reported that the nitrosonium ion induced conversion of alkyl and aryl alkyl halides with nitriles into N-alkyl (arylalkyl) amides in excellent yields. The halohydrocarbons were treated with nitrosonium hexafluorophosphate in nitrile (Scheme 23). The reaction took place with the evolution of nitrosyl halide. The quenching of the reaction mixture with water gives the corresponding amides. The protocol is general and equally applicable to iodides, bromides, chlorides and fluorides.In 2011, Kalkhambkar et al.11 used the [BMIM][PF6]/NOPF6 system for the synthesis of amides via the reaction of nitriles with bromides. In this study, tBu-Br and Ad-Br were used for carbo-cation generation induced by NO+ for reaction with nitriles, which gives the amides in reasonable isolated yields (Scheme 24).
The photo-Ritter reaction is one alternative method that overcomes the disadvantages of the classical Ritter reaction because direct photolysis is a green synthetic method. Only a few examples are found of halo-hydrocarbons as a substrate in photo-Ritter reaction, including alkyl halides, aromatic alkenyl bromides and an aryl halide.29 Bi et al.30 have reported a photo-Ritter reaction of five aryl methyl bromides in acetonitrile. These aryl methyl bromides were dissolved in dry acetonitrile and then irradiated with a 300 W high-pressure lamp or 500 W Xe lamp under a nitrogen atmosphere. The corresponding amides were obtained in good yields (Scheme 25). However, the substrate scope is narrow and only five aryl methyl bromides are involved.
3.5 Ritter-type reaction of liquid carboxylate salts
The imide moiety is an important substructure in natural products. Imides are usually prepared by the reaction of amides with anhydrides, acyl chlorides and carboxylic esters or acids. The synthesis of imides through nitriles and acid anhydrides was introduced by Habibi and co-workers. Khodaei and Nazari31 reported Ritter-type reaction of liquid carboxylate salts for the synthesis of asymmetrical imides using stoichiometric amounts of trifluoromethanesulfonic anhydride (Tf2O, 100 mol%) as a powerful promoter (Scheme 26). In the protocol, three n-butylammonium carboxylates and various nitriles were used in the absence of solvent at 60 °C. Except for benzyl cyanide and 4-nitrobenzyl cyanide, all other nitriles reacted well and imides were obtained in good yields. The main disadvantage of the protocol is the high catalyst loading.The proposed reaction mechanism is shown in Scheme 27. It was thought that mixed anhydrides were first formed by nucleophilic attack of the carboxylate ion on triflic anhydride. The triflate leaving group was then replaced by a nucleophile. The intermediate was hydrolyzed by dilute NaHCO3 solution to yield the corresponding imide.
4. Other Ritter reaction
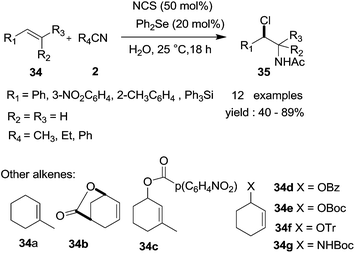
In 2012, Huang et al.32 reported a novel p-toluenesulfonic acid (PTSA, 10 mol%) catalyzed and iodine (100 mol%) mediated head-to-tail dimerization reaction of styrene derivatives and nitriles (Scheme 28). In the presence of p-toluenesulfonic acid and iodine, styrene derivatives undergo head-to-tail dimerization followed by trapping with nitriles to yield the corresponding Ritter-type products. Through this protocol, a series of N-(4-iodo-1,3-diarylbutyl) acetamides were prepared from readily available starting materials in moderate to good yields. However, the electron-rich vinyl arenes reacted reluctantly and by-products were produced.The haloamidation of an alkene is a useful transformation. In 2013, Tay et al.33 described a mild and highly efficient Lewis base catalyzed chloroamidation of alkenes. In the protocol, N-chlorosuccinimide (NCS, 0.5 mol%) and diphenyl selenide (Ph2Se, 20 mol%) were used as the halogenating reagent and catalyst, respectively (Scheme 29). The corresponding products were obtained in moderate to good yields. The reaction conditions are suitable for a wide range of substrates including those which are acid-labile. The proposed mechanism of the protocol is thought to be as follows. The Lewis basic diphenyl selenide activates the chlorine atom on NCS to form an intermediate. The electrophilic Cl may then be delivered to the alkene to form the haliranium intermediate, which undergoes Ritter reaction mechanisms in succession to produce the final products.
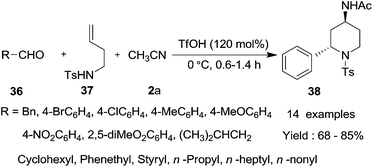
Piperidine derivatives are one of the most promising therapeutic agents for a wide variety of diseases. In 2011, Reddy et al.34 introduced a novel procedure for the synthesis of 4-amidopiperidine derivatives via an aza-Prins–Ritter reaction sequence using triflic acid (TfOH, 120 mol%) as a catalyst in acetonitrile under mild conditions (Scheme 30). A large range of aromatic aldehydes and aliphatic aldehydes underwent smooth coupling with N-tosylhomoallylic amine in acetonitrile to give the corresponding products of 4-amidopiperidine derivatives with trans-selectivity in good yields. The reaction is very fast and no deprotection of tosyl group was observed. However, substituted homoallylamines failed to undergo aza-Prins cyclization under these reaction conditions.
In 2013, Yadav et al.35 introduced a novel method for the synthesis of anti-1,3-aminoalcohols through a highly stereoselective Prins–Ritter reaction followed by reductive opening of the resulting iodomethyl-4-amidotetrahydropyran ring. The key step is the cascade reaction of the Prins–Ritter reaction, which is catalyzed by 20 mol% BF3OEt2 (Scheme 31). The protocol has been successfully applied to the total synthesis of piperidine alkaloids [(−)-halosaline 43, (+)-norallosedamine 44] and β-amino acids 45.
In addition to the piperidine derivatives and 1,3-aminoalcohols, the Ritter reaction has also been used to synthesize aza-bicyclic alkaloids, which have been shown to exhibit broad biological activity and a diverse pharmacological profile. In 2013, Saikia et al.36 reported a simple method for the highly stereo-selective synthesis of aza-bicyclic compounds via a tandem aza-Prins–Ritter/Friedel–Crafts type reaction of endocyclic N-acyliminium ions (Scheme 32). In the presence of boron trifluoride etherate (BF3OEt2, 120 mol%) at room temperature, a series of amido/phenyl-substituted hexahydroindolizin-3(2H)-one, hexahydro-1H-quinolizin-4(6H)-one and 1,3,4,10b-tetrahydropyrido[2,1-a]isoindol-6(2H)-one derivatives were synthesized. Except for the high catalyst loading, the protocol is very atom economic and is promising for the synthesis of other substituted aza-bicyclic alkaloids and in natural product synthesis.
5. Conclusion
This review has shown the spectacular advances that have been made in the Ritter reaction in recent years. The scope of the substrate is expanding at an unprecedented pace. The variety of catalysts and reaction media have been considerably developed. Many useful organic compounds have been synthesized through Ritter or Ritter-type reaction, such as asymmetrical di- and tri-substituted ureas, 3-substituted-3-amino-oxindoles, 4-acyl-aminotetrahydroindazoles, N-(4-iodo-1,3-diarylbutyl) acetamides, 4-amidopiperidine derivatives and aza-bicyclic alkaloids. All these findings make the Ritter reaction very attractive in organic synthesis. However, there are still many problems. First, the high catalyst loading: for most Ritter or Ritter-type reaction the catalyst loading is high (10–30 mol%) and some reactions need stoichiometric amounts of catalyst. Second, the high reaction temperature of most Ritter or Ritter-type reaction. In addition, diastereo-selective Ritter reaction are rare and there has so far been no report of an asymmetric catalyzed Ritter reaction. Therefore efforts toward the use of low catalytic loadings, more facile reaction conditions and enantio-selective catalytic Ritter reaction are now required.Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (21102115), the Spring Plan of Ministry of Education (Z2012020), the Sichuan Education Department (14CZ0013), the National Undergraduate Innovation and the Key Laboratory of XiHua University. We thank Yimou Gong, Zhanwei Cao, Hongjia Zhang, Ke Wei and Ronghua Shi for the early document sorting.References
- (a) J. J. Ritter and P. P. Minieri, J. Am. Chem. Soc., 1948, 70, 4045–4048 CrossRef CAS; (b) J. J. Ritter and J. Kalish, J. Am. Chem. Soc., 1948, 70, 4048–4050 CrossRef CAS.
- (a) S. Stavber, T. S. Pecǎn, M. Papež and M. Zupan, Chem. Commun., 1996, 2247–2248 RSC; (b) V. Nair, R. Rajan and N. P. Rath, Org. Lett., 2002, 4, 1575–1577 CrossRef PubMed; (c) M. Penner and F. Schweizer, Carbohydr. Res., 2006, 342, 7–15 CrossRef PubMed; (d) R. M. A. Pinto, J. A. R. Salvador and C. L. Roux, Synlett, 2006, 2047–2050 Search PubMed; (e) X. Z. Song and R. I. Hollingsworth, Tetrahedron Lett., 2006, 47, 229–232 CrossRef PubMed; (f) L. Castellanos, C. Duque, J. R. Rodríguez and C. Jiménez, Tetrahedron, 2007, 63, 1544–1552 CrossRef CAS PubMed; (g) I. R. Morgan, A. Yazici, S. G. Pyne and B. W. Skelton, J. Org. Chem., 2008, 73, 2943–2946 CrossRef CAS PubMed; (h) M. D. Santos, B. Crousse and D. B. Delpon, Tetrahedron Lett., 2009, 50, 857–859 CrossRef PubMed.
- For recent review on catalytic Ritter reaction, see: (a) A. Guérinot, S. Reymond and J. Cossy, Eur. J. Org. Chem., 2012, 19–28 CrossRef ; For part examples, see ; (b) R. Sanz, A. Martínez, V. Guilarte, J. M. Álvarez-Gutiérrez and F. Rodríguez, Eur. J. Org. Chem., 2007, 4642–4645 CrossRef CAS; (c) T. Yamato, J. Y. Hu and N. Shinoda, J. Chem. Res., 2007, 641–643 CrossRef CAS; (d) M. Barbero, S. Bazzi, S. Cadamuro and S. Dughera, Eur. J. Org. Chem., 2009, 430–436 CrossRef CAS; (e) B. Anxionnat, A. Guérinot, S. Reymond and J. Cossy, Tetrahedron Lett., 2009, 50, 3470–3473 CrossRef CAS PubMed; (f) P. Theerthagiri, A. Lalitha and P. N. Arunachalam, Tetrahedron Lett., 2010, 51, 2813–2819 CrossRef CAS PubMed; (g) E. Callens, A. J. Burton and A. G. M. Barrett, Tetrahedron Lett., 2006, 47, 8699–8701 CrossRef CAS PubMed; (h) H. Firouzabadi, R. Iranpoor and A. Khoshnood, Catal. Commun., 2008, 9, 529–531 CrossRef CAS PubMed; (i) H. Firouzabadi, A. R. Sardarian and H. Badparva, Synth. Commun., 1994, 24, 601–607 CrossRef CAS.
- N. Ibrahim, A. S. K. Hashmi and F. Rominger, Adv. Synth. Catal., 2011, 353, 461–468 CrossRef CAS.
- S. Khaksar, E. Fattahi and E. Fattahi, Tetrahedron Lett., 2011, 52, 5943–5946 CrossRef CAS PubMed.
- S. Q. Jiang, Z. Y. Wang, Z. J. Jiang and J. H. Li, Lett. Org. Chem., 2012, 9, 24–28 CrossRef CAS.
- M. S. Shakeri, H. Tajik and K. Niknam, J. Chem. Sci., 2012, 5, 1025–1032 CrossRef PubMed.
- G. M. Ziarani, A. Badiei, Z. Dashtianeh, P. Gholamzadeh and N. H. Mohtasham, Res. Chem. Intermed., 2013, 39, 3157–3163 CrossRef.
- Recent review about Ionic Liquid, see: (a) I. Toshiyuki, J. Synth. Org. Chem., Jpn., 2014, 72, 518–528 CrossRef; (b) W. G. Cheng, Q. Su, J. Q. Wang and J. Sun, J. Catal., 2013, 3, 878–901 CrossRef PubMed.
- F. Jiang, Y. J. Lin, H. F. Duan, Z. H. Li, J. G. Cao and D. P. Liang, Chem. Res. Chin. Univ., 2010, 26, 384–388 CAS.
- R. G. Kalkhambkar, S. N. Waters and K. K. Laali, Tetrahedron Lett., 2011, 52, 867–871 CrossRef CAS PubMed.
- Reviews about microwave in organic synthesis, see: (a) S. Caddick and R. Fitzmaurice, Tetrahedron, 2009, 65, 3325–3355 CrossRef CAS PubMed; (b) H. M. Hugel, Molecules, 2009, 14, 4936–4972 CrossRef CAS PubMed.
- S. Yaragorla, G. Singh, P. L. Saini and M. K. Reddy, Tetrahedron Lett., 2014, 55, 4657–4660 CrossRef CAS PubMed.
- M. B. Gawande, A. K. Rathi, I. D. Nogueira, R. S. Varma and P. S. Branco, Green Chem., 2013, 15, 1895–1899 RSC.
- H. Basavaprabhu and V. V. Sureshbabu, Org. Biomol. Chem., 2012, 10, 2528–2533 CAS.
- F. Zhou, M. Ding and J. Zhou, Org. Biomol. Chem., 2012, 10, 3178–3181 CAS.
- M. Turks, I. Strakova, K. Gorovojs, S. Belyakov, Y. A. Piven, T. S. Khlebnicova and F. A. Lakhvich, Tetrahedron, 2012, 68, 6131–6140 CrossRef CAS PubMed.
- P. Rubenbauer and T. Bach, Chem. Commun., 2009, 2130–2132 RSC.
- M. H. Al-Huniti and S. D. Lepore, Adv. Synth. Catal., 2013, 355, 3071–3076 CrossRef CAS PubMed.
- B. V. S. Reddy, N. S. Reddy, C. Madan and J. S. Yadav, Tetrahedron Lett., 2010, 51, 4827–4829 CrossRef PubMed.
- Y. Hanzawa, Y. Kasashima, K. Tomono, T. Mino, M. Sakamoto and T. Fujita, J. Oleo Sci., 2012, 61, 715–721 CrossRef CAS.
- M. Mokhtary and G. Goodarzi, Chin. Chem. Lett., 2012, 23, 293–296 CrossRef CAS PubMed.
- M. Mokhtary and F. Najafizadeh, E-J. Chem., 2012, 9, 576–582 CrossRef CAS PubMed.
- V. Panduranga, H. Basavaprabhu and V. V. Sureshbabu, Tetrahedron Lett., 2013, 54, 975–979 CrossRef CAS PubMed.
- For selected recent reviews, see: (a) M. M. Díaz-Requejo and P. J. Peérez, Chem. Rev., 2008, 108, 3379–3394 CrossRef PubMed; (b) F. Collet, R. H. Dodd and P. Dauban, Chem. Commun., 2009, 5061–5074 RSC; (c) F. Collet, C. Lescot and P. Dauban, Chem. Soc. Rev., 2011, 40, 1926–1936 RSC.
- G. A. Olah and B. G. B. Gupta, J. Org. Chem., 1980, 45, 3532 CrossRef CAS.
- Q. Michaudel, D. Thevenet and P. S. Baran, J. Am. Chem. Soc., 2012, 134, 2547–2550 CrossRef CAS PubMed.
- G. A. Olah, B. G. B. J. Gupta and A. C. Narang, Synthesis, 1979, 274–276 CrossRef CAS.
- (a) P. J. Kropp, G. S. Poindexter, N. J. Pienta and D. C. Hamilton, J. Am. Chem. Soc., 1976, 98, 8135–8144 CrossRef CAS; (b) T. Kitamura, S. Kobayashi and H. Taniguchi, Chem. Lett., 1984, 1351–1352 CrossRef CAS; (c) B. Guizzardi, M. Mella, M. Fagnoni and A. Albini, Chem.–Eur. J., 2003, 9, 1549–1555 CrossRef CAS PubMed.
- N. M. Bi, M. G. Ren and Q. H. Song, Synth. Commun., 2010, 40, 2617–2623 CrossRef CAS.
- M. M. Khodaei and E. Nazari, Tetrahedron Lett., 2012, 53, 2881–2884 CrossRef CAS PubMed.
- J. M. Huang, Z. J. Ye, D. S. Chen and H. Zhu, Org. Biomol. Chem., 2012, 10, 3610–3612 CAS.
- D. W. Tay, I. T. Tsoi, J. C. Er, G. Y. C. Leung and Y. Y. Yeung, Org. Lett., 2013, 6, 1310–1313 CrossRef PubMed.
- B. V. S. Reddy, K. Ramesh, A. V. Ganesh, G. G. K. S. N. Kumar, J. S. Yadav and R. Grée, Tetrahedron Lett., 2011, 52, 495–498 CrossRef CAS PubMed.
- J. S. Yadav, Y. J. Reddy, P. A. N. Reddy and B. V. S. Reddy, Org. Lett., 2013, 15, 546–549 CrossRef CAS PubMed.
- K. Indukuri, R. Unnava, M. J. Deka and A. K. Saikia, J. Org. Chem., 2013, 21, 10629–10641 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2014 |