One pot synthesis of 1,2,4,5-tetrasubstituted-imidazoles catalyzed by trityl chloride in neutral media†
Ahmad Reza Moosavi-Zare*a,
Zhila Asgarib,
Abdolkarim Zare*c,
Mohammad Ali Zolfigol*b and
Mohsen Shekouhyd
aDepartment of Chemistry, University of SayyedJamaleddinAsadabadi, Asadabad, 6541835583, Iran. E-mail: moosavizare@yahoo.com
bFaculty of Chemistry, Bu-Ali Sina University, Hamedan, 6517838683, Iran
cDepartment of Chemistry, Payame Noor University, PO Box 19395-3697, Tehran, Iran
dDepartment of Chemistry, College of Science, Shiraz University, 71454, Shiraz, Iran
First published on 3rd November 2014
Abstract
Trityl chloride (TrCl or Ph3CCl) efficiently catalyzes the one-pot multi-component condensation of benzil with aldehydes, primary amines and ammonium acetate under neutral and solvent-free conditions to give 1,2,4,5-tetrasubstituted imidazoles in high to excellent yields and in short reaction times. Mechanistically, it is attractive that trityl chloride promotes the reaction by in situ generation of trityl carbocation (Ph3C+).
Multi-component reactions (MCRs) play an important role in combinatorial chemistry because of their capability to synthesize target molecules with atom economy and high efficiency, by reaction of three or more reactants in one step. Furthermore, these reactions increase synthetic efficacy and simplicity with respect to conventional organic transformations.1
Imidazole derivatives are one of the most important classes of nitrogen-containing five-members heterocycles. For example, they are an essential component of various biological and pharmaceutical molecules, including histidine, histamine, biotin, losartan, olmesartan, eprosartan, miconazole, ketoconazole, clotrimazole and trifenagrel.2 Some pharmaceutical compounds, based on imidazole moiety, are displayed in Fig. 1. Moreover, imidazole derivatives are used as green solvents in form of ionic liquids,3 and as N-heterocyclic carbenes in organometallic chemistry.4 1,2,4,5-Tetrasubstituted imidazoles are an important class of imidazoles which are prepared via the one-pot multi-component condensation of benzil with aldehydes, primary amines and ammonium acetate using acidic catalysts.5 Although several catalysts for this transformation are known, development of newer catalysts with high novelty which can promote the reaction attract attention for their difference with the others, and effectiveness.
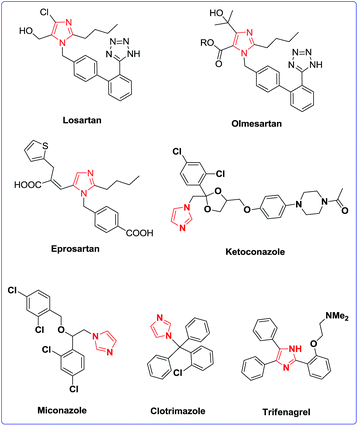 | ||
| Fig. 1 The structure of losartan, olmesartan, eprosartan, miconazole, ketoconazole, clotrimazole and trifenagrel as drugs. | ||
Currently, development of organocatalysts has attracted much attention in organic synthesis, especially from green chemistry point of view.6 These catalysts have some advantages compared with acidic catalysts or precious metal, such as commercial availability, low cost, relative non-toxicity, air stability, need to simple reaction conditions, and ability to promote a chemical reaction via different activation modes.7,8 Considering the high importance of organocatalysts, recently, we have applied a neutral and attractive kind of these catalysts namely triarylmethyl chlorides (Ar3CCl) to promote the preparation of 12-aryl-8,9,10,12-tetrahydrobenzo[a]-xanthen-11-ones,7 bis(indolyl)methanes,9 1-amidoalkyl-2-naphthols10 and N-sulfonyl imines.11 In the mentioned investigations, we have used Ar3CCl to catalyze the two and three-component reactions. However, in the presented work, we employ trityl chloride (TrCl or Ph3CCl) as a homogeneous organocatalyst to promote a one-pot four-component reaction, i.e. the condensation of benzil with aldehydes, primary amines and ammonium acetate, for the first time (Scheme 1).
To optimize the reaction conditions, as a model reaction, the condensation of benzil (1 mmol) with benzaldehyde (1 mmol), aniline (1 mmol) and ammonium acetate (1 mmol) was studied in the presence of different molar ratios of trityl chloride at range of 50–100 °C under solvent-free conditions; the respective results are summarized in Table 1. As Table 1 indicates, the best results were obtained when the reaction was performed using 10 mol% of the organocatalyst at 90 °C. To confirm that heat can not thermodynamically promote the reaction in the absence of catalyst; the model reaction was examined at 90 °C under catalyst-free conditions. In these conditions, the desired product was obtained in trace yield after 120 min. This observation clearly showed that heat can not promote the reaction without catalyst; thus, presence of catalyst (e.g. TrCl) is essential for the reaction.
After optimization of the reaction conditions, the efficiency and scope of the organocatalyst was evaluated by the reaction of benzil with various arylaldehydes, different primary amines and ammonium acetate using 10 mol% of TrCl at 90 °C; the corresponding results are summarized in Table 2. As it can be seen in Table 2, all reactions proceeded efficiently to afford the desired 1,2,4,5-tetrasubstituted imidazoles in high yields and in short reaction times. Thus, TrCl was efficient to catalyze the one-pot four-component reaction.
| Entry | Ar | R | Time (min)/yielda (%) | M.p. (°C) found/reported |
|---|---|---|---|---|
| a Isolated yield. | ||||
| 1a | C6H5 | C6H5 | 30/79 | 218–220/218–221 (ref. 5a) |
| 1b | C6H5 | C6H5CH2 | 32/80 | 156–158/156–159 (ref. 5a) |
| 1c | 4-CH3-C6H4 | C6H5CH2 | 35/76 | 165–167/165–168 (ref. 5a) |
| 1d | 4-Cl-C6H4 | 4-Cl-C6H4 | 20/81 | 188–190/189–191 (ref. 4b) |
| 1f | 3-NO2-C6H4 | 4-CH3-C6H4 | 60/78 | 146–147/145–147 (ref. 4b) |
| 1g | 4-NO2-C6H4 | 4-CH3-C6H4 | 30/81 | 214–217/215–217 (ref. 4b) |
| 1h | 4-Cl-C6H4 | C6H5CH2 | 30/79 | 158–160/161–163 (ref. 4b) |
| 1i | 4-Cl-C6H4 | 4-F-C6H4 | 20/78 | 197–200/198–201 (ref. 4b) |
| 1j | 4-CH3-C6H4 | 4-CH3-C6H4 | 60/75 | 193–195/194–196 (ref. 4b) |
| 1k | 3-CH3O-C6H4 | C6H5CH2 | 40/75 | 131–133/130–132 (ref. 5a) |
| 1l | 4-Cl-C6H4 | C6H5 | 30/79 | 148–150/148–151 (ref. 5a) |
| 1m | 4-OH-C6H4 | C6H5CH2 | 35/78 | 134–137/135–138 (ref. 5a) |
| 1n | 4-CH3O-C6H4 | C6H5CH2 | 45/75 | 158–160/158–161 (ref. 5a) |
| 1o | 4-OH-C6H4 | C6H5 | 35/81 | 280–283/282–284 (ref. 5a) |
| 1p | 4-CN-C6H4 | 4-CH3-C6H4 | 30/82 | 197–200/198–201 (ref. 4b) |
| 1q | 4-OH-C6H4 | 4-CH3-C6H4 | 35/82 | 232–234/233–235 (ref. 5a) |
| 1r | 2-Thienyl | 4-CH3-C6H4 | 20/78 | 198–200/199–202 (ref. 4b) |
| 1s | 2-Thienyl | 4-OH-C6H4 | 3/92 | 198–200/198–201 (ref. 4b) |
In a plausible mechanism which is supported by the literature (Scheme 2),1b,9–11 at first, aldehyde is activated by Ph3C+ generated from Ph3CCl; consequently, two resonance forms of the activated aldehyde (I and II) can produce.1b,9–11 The activated aldehyde react with amine to give iminium intermediate III. Afterward, III reacts with ammonia (resulted from ammonium acetate) to afford IV, and this intermediate reacts with trityl alcohol (Ph3COH) to provide V and Ph3CCl. In the next step, intermediate VI produces by nucleophilic attack of the nitrogens of V to the carbonyl groups of benzil. Finally, 1,2,4,5-tetrasubstituted imidazole is synthesized by removing two molecules of H2O from VI.
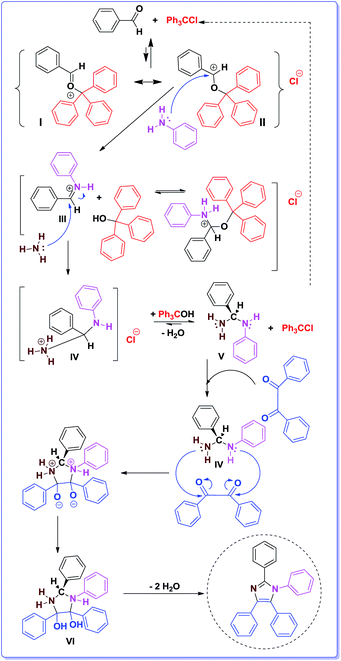 | ||
| Scheme 2 The proposed mechanism for the synthesis of 1,2,4,5-tetrasubstituted imidazoles using trityl chloride. | ||
To confirm the generation of I and II as resonance structures of the activated aldehyde, benzaldehyde was treated with trityl chloride at room temperature, and IR, 1H NMR, and UV spectra of the mixture was run;1b,10 the obtained results include:
IR (nujol): νmax (cm−1) of the carbonyl group of benzaldehyde (1705) was decreased to 1697 in the mixture of benzaldehyde and trityl chloride (Fig. S1†).
1H NMR (300 MHz, CDCl3): chemical shift (ppm) of the HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of benzaldehyde (9.78) was increased to 10.04 in the mixture of PhCHO and TrCl (Fig. S2†).
O of benzaldehyde (9.78) was increased to 10.04 in the mixture of PhCHO and TrCl (Fig. S2†).
UV: maximum absorption of benzaldehyde and trityl chloride were 240 and 222 nm, respectively. However, λmax of the mixture of PhCHO and TrCl was observed in 245 nm (Fig. S3†).
These observations showed that aldehyde in the presence of trityl chloride give resonance forms I and II.
Although TrOH produced during the reaction; but it can not catalyze the reaction like TrCl. To confirm this point, we tested the model reaction in the presence of TrOH as catalyst in which the product yield was only 23% after 50 min. This result verified that TrOH is changed to TrCl during the reaction.
Thus, the mechanism is confirmed by the above observations besides the literature.1b,7,9–11
Catalytic application of other trityl sources such as monomethoxytrityl chloride (Ph2(p-MeOC6H4)CCl, MMTCl), dimethoxytrityl chloride (Ph(p-MeOC6H4)2CCl) and triphenylmethanol (TrOH) on the model reaction, was also investigated under solvent-free conditions at 90 °C. The results are shown in Fig. 2. As Fig. 2 indicates, higher yield and shorter reaction time were obtained using trityl chloride. Furthermore, turn over numbers (TON) and turn over frequencies (TOF) on the effectiveness of these catalysts for the synthesis of the imidazoles were calculated (Fig. 2). The TON and TOF values showed that TrCl is more efficient than MMTCl, DMTCl and TrOH.
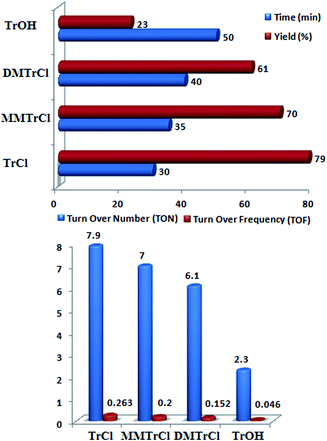 | ||
| Fig. 2 Catalytic activity of different triarylmethyl sources (10 mol%) on the reaction between benzil (1 mmol), benzaldehyde (1 mmol), aniline (1 mmol) and ammonium acetate (1 mmol) at 90 °C. | ||
Conclusions
In summary, we have applied TrCl as a neutral and homogenous organocatalyst for the one-pot four-component reaction of aldehydes with amines (aliphatic or aromatic), benzil and ammonium acetate at 90 °C in solvent-free conditions to furnish 1,2,4,5-tetrasubstituted imidazoles. The promising points for the presented catalytic process include generality, efficacy, high yield, simplicity, cleaner reaction profile, relatively short reaction times and good agreement with the green chemistry protocols.12Acknowledgements
The authors gratefully acknowledge support of this work by the Research Affairs Office of Bu-Ali Sina University (Grant number 32-1716 entitled development of chemical methods, reagents and molecules), University of Sayyed Jamaleddin Asadabadi, Payeme Noor University, and Center of Excellence in Development of Environmentally Friendly Methods for Chemical Synthesis (CEDEFMCS), for providing support to this work.Notes and references
-
(a) Multicomponent Reactions, ed. J. Zhu and H. Bienayme, Wiley, Weinheim, 2005 Search PubMed
; (b) A. Khazaei, M. A. Zolfigol, A. R. Moosavi-Zare, F. Abi, A. Zare, H. Kaveh, V. Khakyzadeh, M. Kazem-Rostami, A. Parhami and H. Torabi-Monfared, Tetrahedron, 2013, 69, 212 CrossRef CAS PubMed
; (c) A. R. Moosavi-Zare, M. A. Zolfigol, S. Farahmand, A. Zare, A. R. Pourali and R. Ayazi-Nasrabadi, Synlett, 2014, 25, 193 CrossRef CAS PubMed
; (d) M. A. Zolfigol, A. Khazaei, A. R. Moosavi-Zare, A. Zare and V. Khakyzadeh, Appl. Catal., A, 2011, 400, 70 CrossRef CAS PubMed
; (e) A. R. Moosavi-Zare, M. A. Zolfigol and M. Daraei, Synlett, 2014, 25, 1173 CrossRef PubMed
.
-
(a) S. A. Laufer, W. Zimmermann and K. J. Ruff, J. Med. Chem., 2004, 47, 6311 CrossRef CAS PubMed
; (b) C. Leister, Y. Wang, Z. Zhao and C. W. Lindsley, Org. Lett., 2004, 6, 1453 CrossRef PubMed
.
- P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis, Wiley-VCH, Weinheim, 2008 Search PubMed
.
-
(a) L. H. Lee, M. Bang and C. S. Pak, Tetrahedron Lett., 2005, 46, 7139 CrossRef PubMed
; (b) A. Hasaninejad, A. Zare, M. Shekouhi and J. Ameri Rad, J. Comb. Chem., 2010, 12, 844 CrossRef CAS PubMed
.
-
(a) M. A. Zolfigol, A. Khazaei, A. R. Moosavi-Zare, A. Zare, Z. Asgari, V. Khakyzadeh and A. Hasaninejad, J. Ind. Eng. Chem., 2013, 19, 721 CrossRef CAS PubMed
; (b) A. Y. Usyatinsky and Y. L. Khmelnitsky, Tetrahedron Lett., 2000, 41, 5031 CrossRef CAS
; (c) S. Kantevari, S. V. N. Vuppalapati, D. O. Biradar and L. Nagarapu, J. Mol. Catal. A: Chem., 2007, 266, 109 CrossRef CAS PubMed
; (d) M. Kidwai, P. Mothsra, V. Bansal, R. K. Somvanshi, A. S. Ethayathulla, S. Dey and T. P. Singh, J. Mol. Catal. A: Chem., 2007, 265, 177 CrossRef CAS PubMed
; (e) B. Sadeghi, B. B. F. Mirjalili and M. M. Hashemi, Tetrahedron Lett., 2008, 49, 2575 CrossRef CAS PubMed
; (f) S. D. Sharma, P. Hazarika and D. Konwar, Tetrahedron Lett., 2008, 49, 2216 CrossRef PubMed
; (g) A. R. Moosavi-Zare, M. A. Zolfigol, V. Khakyzadeh, C. Böttcher, M. H. Beyzavi, A. Zare, A. Hasaninejad and R. Luque, J. Mater. Chem. A, 2014, 2, 770 RSC
.
- A. Dondoni and A. Massi, Angew. Chem., Int. Ed., 2008, 47, 4638 CrossRef CAS PubMed
.
- A. Khazaei, M. A. Zolfigol, A. R. Moosavi-Zare, A. Zare, M. Khojasteh, Z. Asgari, V. Khakyzadeh and A. Khalafi-Nezhad, Catal. Commun., 2012, 20, 54 CrossRef CAS PubMed
.
- S. Verma, L. S. Jain and B. Sain, Tetrahedron Lett., 2010, 51, 6897 CrossRef CAS PubMed
.
- A. Khalafi-Nezhad, A. Parhami, A. Zare, A. R. Moosavi Zare, A. Hasaninejad and F. Panahi, Synthesis, 2008, 617 CrossRef CAS PubMed
.
- A. Khazaei, M. A. Zolfigol, A. R. Moosavi-Zare, A. Zare, A. Parhami and A. Khalafi-Nezhad, Appl. Catal., A, 2010, 386, 179 CrossRef CAS PubMed
.
- A. Khalafi-Nezhad, A. Parhami, A. Zare, A. Nasrollahi Shirazi, A. R. Moosavi-Zare and A. Hasaninejad, Can. J. Chem., 2008, 86, 456 CrossRef CAS
.
- General procedure for the synthesis of 1,2,4,5-tetrasubstituted imidazoles: a mixture of compounds consisting of benzil (1 mmol), aldehyde (1 mmol), primary amine (1 mmol), ammonium acetate (1 mmol) and TrCl (10 mol%) in a 10 mL round-bottomed flask connected to a reflux condenser, was stirred in an oil-bath (90 °C). After completion of the reaction, as monitored by TLC, the reaction mixture was cooled to room temperature, and then petroleum ether (5 mL) was added to the crude reaction mixture, and filtered to separate the catalyst which will be recycled for other reactions. Then, the pure product was obtained by recrystallization of the reaction mixture in ethanol (90%) or ethyl acetate.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra10589c |
| This journal is © The Royal Society of Chemistry 2014 |

