A possible mechanism for the emergence of an additional band gap due to a Ti–O–C bond in the TiO2–graphene hybrid system for enhanced photodegradation of methylene blue under visible light†
Abstract
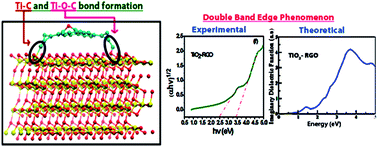
Here we report the experimental and theoretical study of two TiO2–graphene oxide (TG) and TiO2–reduced graphene oxide (TR) composites synthesized by a facile and ecological route, for enhanced visible light (∼470 nm) photocatalytic degradation of Methylene Blue (MB) (99% efficiency), with high rate constant values (1800% over bare TiO2). TG couples TiO2 nanopowder with Graphene Oxide (GO) while TR couples it with reduced graphene oxide (RGO). The present study, unlike previous reports, discusses never-before-reported double absorption edges obtained for both TG (3.51 eV and 2.51 eV) and TR (3.42 eV and 2.39 eV) composites, which represents the reason behind feasible visible light (2.56 eV) induced photocatalysis. TiO2 domains in the composites dominate the higher band edge, while GO/RGO domains explain the lower band edge. Formation of Ti–O–C bonds in both TG and TR drives the shifting upwards of the valence band edge and reduction in band gap. Further, these bonds provide a conductive pathway for charge carriers from TiO2 nanopowder to the degraded species via the GO/RGO matrix, resulting in decreased charge carrier recombination in TiO2 and enhanced efficiency. To attest that the developed theory is correct, density function theory (DFT) calculations were performed. DFT obtained energetics and electronic structures support experimental findings by demonstrating the role of the Ti–O–C bond, which results in double band edge phenomenon in composites. Finally, the mechanism behind MB degradation is discussed comprehensively and the effect of the weight percent of GO/RGO in the composite on the rate constant and photodegradation efficiency has been studied experimentally and explained by developing analytical equations.

 Please wait while we load your content...
Please wait while we load your content...