K2CO3-mediated, direct C–H bond selenation and thiolation of 1,3,4-oxadiazoles in the absence of metal catalyst: an eco-friendly approach†
Jamal Rafiquea,
Sumbal Sabaa,
Alisson R. Rosárioa,
Gilson Zenib and
Antonio L. Braga*a
aDepartamento de Química, Universidade Federal de Santa Catarina, Florianópolis 88040-900, SC, Brazil. E-mail: braga.antonio@ufsc.br; Fax: +55 48 3721 6427; Tel: +55 48 37216427
bDepartamento de Química, Universidade Federal de Santa Maria, Santa Maria 97105-900, RG, Brazil
First published on 2nd October 2014
Abstract
An eco-friendly, straightforward and high-yielding methodology for the synthesis of chalcogenyl oxadiazoles via the K2CO3-promoted direct C–H bond chalcogenation of 2-substituted-1,3,4-oxadiazoles is described herein. The reaction was performed in the absence of metal catalyst and inert atmosphere using only half an equiv. of dichalcogenide and a low-cost base.
Metal-free reactions can be applied in the functionalization of C–H bonds to access C–C and C–heteroatom bonds and this has become a rapidly developing area.1 In this regard, one of the most important discoveries made in organic synthesis in recent years is that certain reactions which were thought to involve transition metal (TM) catalysis can, in fact, proceed without the requirement for a TM.2 Reactions carried out under metal-free conditions are particularly attractive in the synthesis of pharmaceuticals.3 Therefore, from the economic and environmental viewpoints, it would be advantageous and desirable to develop TM-free systems in the area of organic synthesis.
The synthetic versatility of organochalcogenides has been explored extensively in research articles,4 reviews5 and books.6 This group includes the organoselenium compounds, which can be employed in certain reactions7 as catalysts,8 ionic liquids,9 and synthetic intermediates in total synthesis.5,6,10 Another important advancement in this context is the formation of C–Se bonds, which has contributed to the synthesis of a wide range of biologically active molecules11 and functional materials.12 A large number of organoselenides have been found to function as antioxidants, antinociceptive agents, antidepressant apoptosis inducers and chemopreventors in several organs, etc.5d–e,11
Functionalization of the 1,3-4-oxadiazoles scaffold is an important synthetic task, since oxadiazoles are well established as “privileged scaffolds” and are widely used for pharmaceutical, biological and material applications.13 They show a very broad spectrum of biological activity, being used, for instance, as inhibitors of various enzymes and as antimicrobial, analgesic, antiviral and antitumor agents.14 Interestingly, few of the active compounds have a sulphur linkage at C-5.14b 1,3,4-Oxadiazole motifs are also of interest in material science and have been widely used to create novel materials.15
Many methods for the C–H functionalization of 1,3,4-oxadiazoles have been reported in the literature with the formation of C-alkyl,16a C-allyl,16b C-alkynyl,16c C-aryl,16d C-benzyl,16e C–N,16f C–S,16g–h etc. However, the disadvantages associated with many of these methodologies, owing to the use of TM catalysts, expensive reagents, harsh reaction conditions, hazardous materials, oxygen-free techniques or elaborate multi-stepped processes, have limited their synthetic scope.
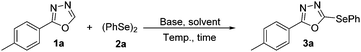
Considering the significance of these compounds, the challenging task of developing new green routes for the syntheses of chalcogenides which provide high efficiency, through direct substitution with heteroaromatics and other organic moieties, is an important research area.17 As part of our wider research program aimed at designing and developing eco-friendly processes,18 herein we report for the first time a straightforward, mild, and environmentally benign protocol for the direct selenation of 1,3,4-oxadiazole, which is also applicable to disulphides. The functionalization of Csp2–H bonds proceeded smoothly with half equiv. of different dichalcogenides and a low-cost base in the absence of a metal catalyst and in an inert atmosphere.
To identify the best reaction conditions, 2-(4-methylphenayl)-1,3,4-oxadiazole (1a) and diphenyl diselenide (2a) were initially used as standard substrates under different conditions, Table 1. Considering the need for a metal catalyst and base under inert atmosphere for Csp2–H bond funtionalization,17b a preliminary experiment was performed using 1 equiv. of K2CO3 and 20 mol% of CuO-nanopowder under an inert atmosphere in DMSO (Table 1, entry 1), affording the product 3a in 56% yield. Notably, the yield was 58% under catalyst-free conditions (entry 2). However, there was a slight improvement in the yield under an open atmosphere (Table 1, entry 3). These results suggest that the use of TM-free and open atmosphere conditions is appropriate for the selenation of 2a.
| Entry | Base | Solvent | Temp. (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.5 mmol), 2a (0.26 mmol), base (0.5 mmol), solvent (1 ml) at a specific time under air.b Isolated yield based on 1a.c CuO-nano (20 mol%).d Under argon atmosphere.e 2a (0.5 mmol).f K2CO3 (2 equiv.).g K2CO3 (0.5 equiv.).h K2CO3 (30 mol%).i K2CO3 with 99.997% purity. | |||||
| 1c,d | K2CO3 | DMSO | 100 | 18 | 56 |
| 2d | K2CO3 | DMSO | 100 | 18 | 58 |
| 3 | K2CO3 | DMSO | 100 | 18 | 63 |
| 4 | K2CO3 | DMSO | 100 | 12 | 81 |
| 5 | K2CO3 | DMSO | 100 | 10 | 86 |
| 6 | K2CO3 | DMSO | 100 | 8 | 82 |
| 7 | K2CO3 | DMSO | 120 | 10 | 59 |
| 8 | K2CO3 | DMSO | 80 | 10 | 71 |
| 9 | K2CO3 | DMSO | rt | 10 | 9 |
| 10e | K2CO3 | DMSO | 100 | 10 | 85 |
| 11 | K2CO3 | DMSO-dry | 100 | 10 | 84 |
| 12 | K2CO3 | DMF | 100 | 10 | 67 |
| 13 | K2CO3 | H2O | 100 | 10 | 0 |
| 14 | K2CO3 | THF | 100 | 10 | 0 |
| 15 | K2CO3 | EtOH | 100 | 10 | 0 |
| 16 | K2CO3 | Toluene | 100 | 10 | 11 |
| 17 | K2CO3 | Acetonitrile | 100 | 10 | 12 |
| 18 | K2CO3 | 1,4-Dioxane | 100 | 10 | 0 |
| 19 | — | DMSO | 100 | 10 | 0 |
| 20 | Na2CO3 | DMSO | 100 | 10 | 59 |
| 21 | Cs2CO3 | DMSO | 100 | 10 | 32 |
| 22 | t-BuOK | DMSO | 100 | 10 | 0 |
| 23 | Et3N | DMSO | 100 | 10 | 0 |
| 24 | KOH | DMSO | 100 | 10 | 13 |
| 25f | K2CO3 | DMSO | 100 | 10 | 82 |
| 26g | K2CO3 | DMSO | 100 | 10 | 61 |
| 27h | K2CO3 | DMSO | 100 | 10 | 41 |
| 28i | K2CO3 | DMSO-dry | 100 | 10 | 85 |
In the next step the reaction time and temperature were screened for this transformation (entries 4–9). Ideal conditions were found to be 10 h reaction time at 100 °C, resulting in 3a with 86% yield (entry 5). It should be noted that increasing the stoichiometric amount of 2a to 0.5 mmol did not affect the yield of 3a (entry 10), while the unreacted 2a was completely recovered using column chromatography. During the course of the reaction the formation of PhSePh, a common by-product, was not observed.
Subsequently, using freshly distilled and dried DMSO was found to be ineffective and the product was obtained in 84% yield (entry 11). Other solvents were also screened and they failed to provide a more favourable outcome (entries 12–18). No product was observed in the absence of base (entry 19). The influence of other bases was also investigated (entries 20–24), all giving poor results. An adverse effect on the yield was noted when the amount of base (K2CO3) varied from 1 mmol (entries 25–27). Finally, in order to eliminate any possibility of a catalytic effect due to trace metal contamination,17b,19 a controlled experiment was carried out using K2CO3 with 99.997% purity, freshly distilled DMSO, new glassware and a new magnetic bar (entry 28). This experiment afforded 3a with 85% yield and thus we can assume that a trace metal was not involved in these reactions.
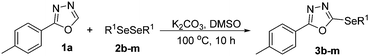
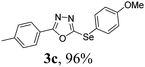
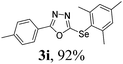
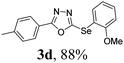
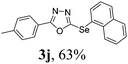
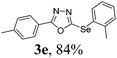
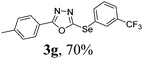
After determining the ideal reaction parameters, we explored the efficiency and generality of our methodology by applying it to various diorganyl diselenides 2 under the optimized conditions (Table 2). The reaction worked well for several diselenides containing both electron-donating (R1 = Me, OMe) and electron-withdrawing (R1 = F, Cl, CF3) groups as well as bulky groups, verifying the sensitivity and tolerance to electronic effects and steric effects of several different substituents. Generally, electron-donating groups at the phenyl ring of 2 afforded good results (entries 1–4 vs. 5–7). There was a weaker influence on the yields due to steric hindrance of ortho-substituted aryl substrates (entries 1 and 2) as compared to the respective para derivatives (entries 3 and 4). Sterically-hindered substrates (R1 = 2-napthyl) resulted in the desired product 3j in 63% yield (entry 9), but dimesityldiselenide not only showed complete tolerance but afforded 3i with 92% yield (entry 8). Normally, aliphatic diselenides are considered to be less reactive and give low yields due to β-selenoxide elimination.17b Gratifyingly, under our optimized conditions, aliphatic diselenides produced the corresponding products in very good yields (entries 10 and 11). We were also delighted to find that C-2 heteroaryl diselenide afforded the desired product 3m with 79% yield (entry 12).
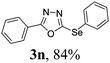
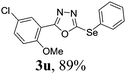
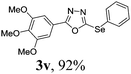
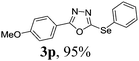
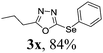
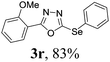
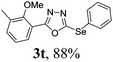
To further extend the scope of the reaction in relation to the substrate, we tested various 2-subtituted 1,3,4-oxaidazoles 1 (Table 3). All of these substrates provided the desired product from good to excellent yields. The electronic and steric properties of the substituents in the benzene ring exerted a very limited influence on the reactivity (entries 1–9). Substrates with di- and tri-substitutions on the phenyl ring, participated effectively to give the desired product 3t–v. These results encouraged us to examine the heteroaromatic and aliphatic groups at the C-2 position of oxadiazole and the desired products were also obtained in very good yields (Table 3, entries 10 and 11). Interestingly, in an attempt to use bis-1,3,4-oxaidzoel as the substrate, the selenated product 3y was obtained in good yield (entry 12), a compound which is a potential candidate for use in material sciences.20
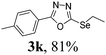
In order to further investigate the scope of this new methodology we extended our study to disulphides (Scheme 1), applying the optimal reaction conditions (Table 1, entry 5). Interestingly, the reaction of different diaryl disulphides 4a–b with oxadiazole 1a proceeded smoothly and afforded the corresponding C-5 thiolated oxadiazoles 5a and 5b in 55% and 49% isolated yield (Scheme 1). The decrease in the yield values can be explained by the stronger S–S bond of diaryl disulphides compared to the respective diselendies.
To demonstrate the synthetic utility of this new protocol, a series of reactions was carried out on different scales (Fig. 1; up to 10 mmol). Oxadiazole 1a and diselenide 2a were selected as the test materials, affording 3a with no major decrease in yield. Therefore, this method could be used as a practical method to synthesize biologically-relevant lead compounds.
To elucidate the mechanism, some control experiments were conducted (Scheme 2). The radical inhibitor (TEMPO) did not hamper the reaction and 3a was obtained in 74% yield [eqn (1)], which excluded the possibility of a radical pathway. 3a was obtained with 71% yield when phenyl selenium bromide was used instead of diphenyl diselenide 2a [eqn (2)], demonstrating that the reaction passes through a phenyl selenium cation species. While under an inert atmosphere, the reaction afforded 3a with 76% yield [eqn (3)], indicating that although oxygen is not required it can facilitate the reaction. Subsequently, improvement in the yield of 3a was observed when 2a was added after 10 min under standard conditions [eqn (4)], suggesting that deprotonation of the oxadiazole core is an important step in the reaction.
Based on these results, a plausible mechanism is proposed in Scheme 3 as follows. Initially, the K2CO3/base-promoted deprotonation of the oxadiazole core takes place at the C-5 position, generating an anion. Subsequently, the selenated product is formed through nucleophilic attack of the anion on diorganyldiselenide, while the organo-selenolated species (anionic) is oxidized back to diselenide.
In conclusion, we developed an efficient, economical and greener K2CO3-promoted procedure for the synthesis of selenated and thiolated oxadiazoles through Csp2–H bond functionalization, in the absence of metal catalyst. We prepared for the first time selenated oxadiazoles, compounds with potential for biological applications. Under mild conditions the reaction worked well in the presence of K2CO3, with a half equiv. of diorganyl dichalcogenides, without the exclusion of air and moisture, affording a wide range of chalcogenated oxadiazoles at the C-5 position in good to excellent yields. The various substituents with different electronic effects and steric effects tolerated the optimized reaction conditions.
The chemistry described herein represents a feasible eco-friendly synthetic approach for the preparation of chalcogenated oxadiazoles through the C–S/Se bond. This novel method provides a complementary, environmentally benign, and easy-operation approach to accessing 5-chalcogenayloxadiazole derivatives.
Acknowledgements
We gratefully acknowledge CNPq, TWAS, INCT-Catálise and FAPESC-Pronex for financial support. J.R. and S.S. thank the CNPq and TWAS for doctoral fellowships. The authors are also grateful to CEBIME for the HRMS analysis.Notes and references
- (a) Y. Xu, X. Tang, W. Hu, W. Wu and H. Jiang, Green Chem., 2014, 16, 3720 RSC; (b) Y. Siddaraju, M. Lamani and K. R. Prabhu, J. Org. Chem., 2014, 79, 3856 CrossRef CAS PubMed; (c) K. Matcha and A. P. Antonchick, Angew. Chem., Int. Ed., 2013, 52, 2082 CrossRef CAS PubMed; (d) S. Wertz, D. Leifert and A. Studer, Org. Lett., 2013, 15, 928 CrossRef CAS PubMed.
- (a) J. Cuthbertson, V. J. Gray and J. D. Wilden, Chem. Commun., 2014, 50, 2575 RSC; (b) C.-L. Sun, H. Li, D.-G. Yu, M. Yu, X. Zhou, X.-Y. Lu, K. Huang, S.-F. Zheng, B. J. Li and Z.-J. Shi, Nat. Chem., 2010, 2, 1044 CrossRef CAS PubMed; (c) M. Rueping, M. Leiendecker, A. Das, T. Poisson and L. Bui, Chem. Commun., 2011, 47, 10629 RSC.
- (a) X.-Q. Chu, H. Meng, Y. Zi, X.-P. Xu and S.-S. Ji, Chem. Commun., 2014, 50, 9721 Search PubMed; (b) X. Zhang, M. Wang, P. Lia and L. Wang, Chem. Commun., 2014, 50, 8006 RSC; (c) H. J. Kim, J. Kim, S. H. Cho and S. Chang, J. Am. Chem. Soc., 2011, 133, 16382 CrossRef CAS PubMed.
- (a) S. Thurow, F. Penteado, G. Perin, R. G. Jacob, D. Alves and E. J. Lenardão, Green Chem., 2014, 16, 3854 RSC; (b) D. S. Rampon, L. A. Wessjohann and P. H. Schneider, J. Org. Chem., 2014, 79, 5987 CrossRef CAS PubMed; (c) C. Santi, B. Battistelli, L. Testaferria and M. Tiecco, Green Chem., 2012, 14, 1277 RSC; (d) V. G. Ricordi, C. S. Freitas, G. Perin, E. J. Lenardão, R. G. Jacob, L. Savegnago and D. Alves, Green Chem., 2012, 14, 1030 RSC; (e) C. S. Freitas, A. M. Barcellos, V. G. Ricordi, J. M. Pena, G. Perin, R. G. Jacob, E. J. Lenardão and D. Alves, Green Chem., 2011, 13, 2931 RSC.
- (a) B. Godoi, R. F. Schumacher and G. Zeni, Chem. Rev., 2011, 111, 2937 CrossRef CAS PubMed; (b) G. Zeni, D. S. Lüdtke, R. B. Panatieri and A. L. Braga, Chem. Rev., 2006, 106, 1032 CrossRef CAS PubMed; (c) G. Perin, E. J. Lenardão, R. G. Jacob and R. B. Panatieri, Chem. Rev., 2009, 109, 1277 CrossRef CAS PubMed; (d) K. P. Bhabak and G. Mugesh, Acc. Chem. Res., 2010, 43, 1408 CrossRef CAS PubMed; (e) C. W. Nogueira, G. Zeni and J. B. T. Rocha, Chem. Rev., 2004, 104, 6255 CrossRef CAS PubMed.
- (a) The Chemistry of Organic Selenium and Tellurium Compounds, ed. Z. Rappoport, Wiley & Sons, Ltd, Chichester, 2014, vol. 4 Search PubMed; (b) Handbook of Chalcogen Chemistry: New Perspectives in Sulfur, Selenium and Tellurium, ed. F. A. Devillanova and W.-W. du Mont, RSC, Cambridge, 2nd edn, 2013 Search PubMed; (c) Organoselenium Chemistry: Synthesis and Reactions, ed. T. Wirth, Wiley-VCH, Weinheim, 2011 Search PubMed.
- (a) M. Godoi, M. W. Paixão and A. L. Braga, Dalton Trans., 2011, 40, 11347 RSC; (b) A. L. Braga, D. S. Lüdtke, J. A. Sehnem and E. E. Alberto, Tetrahedron, 2005, 61, 11664 CrossRef CAS PubMed; (c) Y. Nishiyama, A. Katsuura, A. Negoro and S. J. Hamanaka, J. Org. Chem., 1991, 56, 3776 CrossRef CAS.
- (a) E. E. Alberto, A. L. Braga and M. R. Detty, Tetrahedron, 2012, 68, 10476 CrossRef CAS PubMed; (b) F. V. Singh and T. Wirth, Org. Lett., 2011, 13, 6504 CrossRef CAS PubMed.
- (a) S. Thurow, N. T. Ostosi, S. R. Mendes, R. G. Jacob and E. J. Lenardão, Tetrahedron Lett., 2012, 53, 2651 CrossRef CAS PubMed; (b) H. S. Kim, Y. J. Kim, H. Lee, K. Y. Park, C. Lee and C. S. Chin, Angew. Chem., Int. Ed., 2002, 41, 4300 CrossRef CAS.
- (a) J.-D. Yu, W. Ding, G.-Y. Lian, K.-S. Song, D.-W. Zhang, X. Gao and D. J. Yang, J. Org. Chem., 2010, 75, 3232 CrossRef CAS PubMed; (b) K. C. Nicolaour, D. J. Edmonds and P. G. Bulger, Angew. Chem., Int. Ed., 2006, 45, 7134 CrossRef PubMed; (c) C. C. Silveira, A. Machado, A. L. Braga and E. J. Lenardão, Tetrahedron Lett., 2004, 45, 4077 CrossRef CAS PubMed.
- A. L. Braga and J. Rafique, in The Chemistry of Organic Selenium and Tellurium Compounds, ed. Z. Rappoport, Wiley & Sons, Ltd, Chichester, 2014, vol. 4, ch. 13–15, pp. 989-1174 Search PubMed.
- (a) T. E. Frizon, D. S. Rampon, H. Gallardo, A. A. Merlo, P. H. Schneider, O. E. D. Rodrigues and A. L. Braga, Liq. Cryst., 2012, 39, 769 CrossRef CAS; (b) S. Haid, A. Mishra, M. Weil, C. Uhrich, M. Pfeiffer and P. Bäuerle, Adv. Funct. Mater., 2012, 22, 4322 CrossRef CAS.
- E. Maccioni, S. Alcaro, R. Cirilli, S. Vigo, M. C. Cardia, M. L. Sanna, R. Meleddu, M. Yanez, G. Costa, L. Casu, P. Matyus and S. Distinto, J. Med. Chem., 2011, 54, 6394 CrossRef CAS PubMed.
- (a) J. Boström, A. Hogner, A. Llinàs, E. Wellner and A. T. Plowright, J. Med. Chem., 2012, 55, 1817 CrossRef PubMed; (b) C. S. Oliveira, B. F. Lira, V. S. F. Silva, J. P. Siqueira Jr, J. M. Barbosa-Filho and P. F. Athayde-Filho, Molecules, 2012, 17, 5095 CrossRef PubMed; (c) M. A. Khanfar, R. A. Hill, A. Kaddoumi and K. A. El Syed, J. Med. Chem., 2010, 53, 8535 CrossRef PubMed.
- (a) J. Lü, Z. Ma, B. Meng, D. Sui, B. Zhang, Z. Xie, X. Jing, F. Wang, J. Ding and L. Wang, Opt. Express, 2011, 19, A1241 CrossRef PubMed; (b) S. Varghese, N. S. S. Kumar, A. Krishna, D. S. S. Rao, S. K. Prasad and S. Das, Adv. Funct. Mater., 2009, 19, 2064 CrossRef; (c) S.-H. Hsiao and G.-S. Liou, Polym. J., 2002, 34, 917 CrossRef CAS.
- (a) L. Chu and F.-L. Qing, J. Am. Chem. Soc., 2012, 134, 1298 CrossRef CAS PubMed; (b) B. Das, G. C. Reddy, P. Balasubramanyam and N. Salvanna, Tetrahedron, 2012, 68, 300 CrossRef CAS PubMed; (c) T. Kawano, N. Matsuyama, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2010, 75, 1764 CrossRef CAS PubMed; (d) C. Li, P. Li, J. Yang and L. Wang, Chem. Commun., 2012, 48, 4214 RSC; (e) T. Mukai, K. Hirano, T. Satoh and M. Miura, Org. Lett., 2010, 12, 1360 CrossRef CAS PubMed; (f) S. Wertz, S. Kodama and A. Studer, Angew. Chem., Int. Ed., 2011, 50, 11511 CrossRef CAS PubMed; (g) S. H. Wunderlich and P. Knochel, Angew. Chem., Int. Ed., 2007, 46, 7685 CrossRef CAS PubMed; (h) L.-H. Zou, J. Reball, J. Mottweiler and C. Bolm, Chem. Commun., 2012, 48, 11307 RSC.
- (a) P. Sang, Z. Chen, J. Zou and Y. Zhang, Green Chem., 2013, 15, 2096 RSC; (b) A. R. Rosario, K. K. Casola, C. E. S. Oliveira and G. Zeni, Adv. Synth. Catal., 2013, 355, 2960 CrossRef CAS.
- (a) M. Godoi, E. W. Ricardo, G. V. Botteselle, F. Z. Galetto, J. B. Azerado and A. L. Braga, Green Chem., 2012, 4, 456 RSC; (b) M. Godoi, G. V. Botteselle, J. Rafique, M. S. T. Rocha, J. M. Pena and A. L. Braga, Asian J. Org. Chem., 2013, 2, 746 CrossRef CAS; (c) D. Singh, S. Narayanaperumal, K. Gul, M. Godoi, O. E. D. Rodriques and A. L. Braga, Green Chem., 2010, 12, 957 RSC.
- N. E. Leadbeater, Nat. Chem., 2010, 2, 1007 CrossRef CAS PubMed.
- R. Cristiano, D. M. P. O. Santos and H. Gallardo, Liq. Cryst., 2004, 32, 7 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Details on the experimental procedure and characterization, as well as the spectral data for all synthesized compound. See DOI: 10.1039/c4ra10490k |
| This journal is © The Royal Society of Chemistry 2014 |