Reaction induced phase separation in thermosetting/thermosetting blends: effects of imidazole content on the phase separation of benzoxazine/epoxy blends†
Pei Zhao,
Qian Zhou,
Yu Yuan Deng,
Rong Qi Zhu and
Yi Gu*
College of Polymer Sciences and Engineering, State Key Laboratory of Polymer Materials Engineering, Sichuan University, Chengdu, China. E-mail: guyi@scu.edu.cn; Fax: +86-28-85405138; Tel: +86-28-85405138
First published on 11th November 2014
Abstract
A micro-sized phase separation structure was successfully realized in normally homogeneous benzoxazine (BZ)/epoxy (ER) (mass ratio: 80/20) blending systems in situ by enhancing the content of catalyst, imidazole (MZ). To reveal the internal relationships between the content of MZ and the phase separation, the phase morphology, polymerization activity, rheology and relaxation behaviors of BZ/ER blends with different contents of MZ were investigated by means of turbidity observation, scanning electron microscopy (SEM), differential scanning calorimetry (DSC), and rheological and dynamic mechanical thermal analysis (DMA), respectively. The results showed that in the BZ/ER/MZ blending system, when the content of MZ was high enough (≥8 wt%), micro-sized ER-rich domains could be obtained, otherwise a homogeneous structure was obtained. What's more, the curing reaction of BZ/ER/MZ blends at 110 °C was mainly associated with the polymerization of ER with MZ. Increasing the MZ content not only increased the reactivity difference between ER and BZ and accelerated the gelation, but also resulted in branched or looser ER networks and relatively low system viscosity, therefore was beneficial to phase separation.
1. Introduction
In the past few decades, thermosetting (TS)/thermosetting (TS) blending systems have attracted more and more attention due to their wide applications in adhesives, filler-reinforced composite materials, automotive and aircraft components, and coatings for electronic circuits.1,2 However, because of the highly crosslinked network structures, TS/TS blending systems are brittle materials that show unsatisfying toughness and elongation at break. Effective toughening strategies are of high interest to enable powerful new applications. It is well known that the properties of polymer blends are not only associated with their chemical structures but also to their phase structures.3–5 For multi-component polymer systems, the introduction of multiphase structures can greatly improve the toughness of the products without sacrificing the other excellent properties, such as modulus and thermal mechanical properties, which is an interesting modification method used in the polymer industry.6 Therefore the introduction of multiphase structures in the TS/TS blends was supported to be a good way to improve the toughness.As one of the method to introduce multiphase structures in multi-component polymer systems, reaction-induced phase separation, which is designed as the uniform precursor system undergoing a phase separation into a two phase separated structure during the development of the curing process, has attracted much attention. This method has been widely used in the thermoplastic (TP) modified TS resin systems,3,7–9 but was fewly reported in TS/TS blending systems.10 For TS/TP blends, such as epoxy/poly(etherimide) blends,11 because there is much difference between TP resin and TS precursor on their thermodynamic compatibility and initial dynamic asymmetry (such as, molecular weight (Mn), viscosity and glass transition temperature (Tg)), the entropy of mixing as well as the compatibility can be significantly reduced with the curing reaction of TS resin.12 While for TS/TS blends, such as benzoxazine/epoxy blending system,13 because the two components have good compatibility and are initial dynamic symmetry (e.g. similar low Mn, low viscosity and Tg), the initial entropy of mixing is huge, which makes it difficult to get phase separation structure. In addition, in TS/TS blends, mutual entanglement of polymer chains in the cross-linked networks or the copolymerization between the two components always leads to the formation of permanent interlocked homogeneous structures.
Accordingly, increasing the dynamic asymmetry during the curing reaction process and reducing the mutual entanglement as well as the copolymerization reaction are promising strategies to get phase separation structures in TS/TS blending systems. Among the ways to increase the dynamic asymmetry, sequential polymerization of different component in the blending systems which has been reported previously, will be a good choice.14–16 The sequential polymerization refers to that one component (component A) can polymerize to a certain degree before another one (component B) starts to polymerize in the presence of curing reagent. During this process, the Mn difference between the two components can be gradually enlarged and the systemic entropy of mixing can be significantly decreased, which will promote the phase separation of component A from matrix B. Through this method, Yang and Dean etc. have obtained phase separation structures in urethane/acrylate resin and epoxy/methacrylic acid blending systems, respectively.14,15 However, it should be noted that in their systems, the polymerization mechanisms between the two components are largely different (e.g. photo-initiated and thermal-initiated) and no copolymerization between the two components exists.
Nevertheless, it is well known that the occurrence of phase separation in multi-component blending system depends on the competition effect between the kinetics of thermodynamic driving force for phase separation and cross-linking reaction of the polymerization component.7 In TS/TS (A/B) blending system, the preferential polymerization of A resin will increase the Mn of polymer A, thus enlarge the dynamic asymmetry between A and B resin and increase the thermodynamic driving force for phase separation, which is beneficial to phase separation. On the other hand, the crosslinking reaction of A resin will lead to vitrification and the increment of viscosity of A/B blending system, which will inhibit phase separation. Therefore, how to balance the opposite effects of curing reaction of A resin on the phase separation is the key to get multi-phase separation structures in TS/TS blending systems.
Due to the good processability, excellent thermal and physical mechanical properties, benzoxazine (BZ)/epoxy (ER) blending system has attracted much more attention in the past decades.17 Herein, BZ/ER blending system is chosen as an example to study the phase separation of TS/TS blending systems. Ishida and Rimdusit et al. studied the curing behaviour of BZ/ER binary blending system and reported that the phenolic hydroxyl groups (–OH) produced by the ring-opening polymerization of BZ could initiate the polymerization of epoxy groups,13,18–20 leading to the copolymerization between BZ and ER. Besides, the curing mechanisms of BZ and ER resin both belong to ring-opening mechanism,21,22 most of the ER polymerization catalysts (or initiators), such as Lewis acids,23,24 imidazole,25–27 tertiary amine28,29 and organic acids,30 etc., can also be used to catalyze the polymerization of BZ resin.31–37 Consequently, it was relatively difficult to precisely control the polymerization sequence of BZ and ER resin via catalyst design and no phase separation phenomenon was reported in BZ/ER blending system.13,38–43 Until recently, Grishchuk et al. occasionally observed nano-sized phase domains in BZ/ER/amine blending systems by atomic force microscope.34,44 However, no systematic investigations about the phase separation of the blending systems and their influencing factors were reported.
Our group has done a lot of contributions to develop phase separation structures in BZ/ER blends. We investigated the possibility of phase separation in BZ/ER blends by model component, and concluded that if epoxy resin can polymerize to long molecular chains before the polymerization of BZ resin, phase separation could be observed in BZ/ER blends.45 Besides, based on the long terms investigation about the catalytic polymerization of BZ and ER resin, we found that imidazole (MZ), a low temperature curing agent of ER resin, is a proper catalyst to realize the sequential polymerization of ER and BZ at proper initial curing temperatures (80 °C–140 °C).46 Recently, using MZ as catalyst, we successfully prepared sea-island phase morphology in BZ/ER blending systems at 110 °C via reaction-inducted phase separation method.47
To be honest, BZ/ER/MZ blending system has been investigated for a long term, but only homogeneous structure was reported.46 Comparing the reported systems with our novel multiphase systems, we find that in the same BZ and ER mass ratio, MZ content is the critical factor that influencing the final structure. In this work, we devoted ourselves to reveal the internal relationship between MZ content and phase separation in BZ/ER/MZ blending systems. In order to do this, the influence of MZ content on the phase morphology, curing reactive of BZ and ER, and rheological behaviour were systematically investigated by means of differential scanning calorimetry (DSC), turbidity observation, scanning electron microscope (SEM), Fourier transform infrared (FTIR), rheological measurement and dynamic mechanical thermal analysis (DMA).
2. Experimental
2.1 Materials
Benzoxazine resin (BZ), industrial grade, based on 4,4′-diaminodiphenyl methane (DDM), phenol and paraformaldehyde was synthesized and characterized as previous report,48 and was used without further purification. The average number molecular weight (Mn) of BZ resin was about 435 g mol−1. Phenol, DDM and paraformaldehyde were purchased commercially from Chengdu Kelong Chemical Reagents Corp. (China) and used as received. Bisphenol-A-based ER (commercial name E44), industrial grade, was provided by Shanghai synthetic resin factory (China) and had an epoxy equivalent weight of 227–246 g eq−1. Phenol, DDM, paraformaldehyde, tetrahydrofuran (THF) and imidazole (MZ, melting temperature 91 °C) were supplied by Chengdu Kelong Chemical Reagents Corp. (China). The chemical structures of materials were shown in Scheme 1.2.2 Preparation of BZ/ER/MZ blends
The blends of BZ/ER/MZ were prepared by mixing BZ and ER resin (mass ratio: 80/20) at 110 °C for 20 min, and when the mixture was cooled to 95 °C, MZ was added and the mixture was stirred vigorously for 2 min until MZ completely dissolved. The samples were degassed under vacuum for another few minutes afterward and then refrigerated (−18 °C) to avoid any further curing reaction. Samples for BZ/MZ and ER/MZ were prepared by the same way.The blends of BZ/ER/MZ involved in this work were defined as BZ/ER/MZ (3 wt%), BZ/ER/MZ (8 wt%) and BZ/ER/MZ (12 wt%). Herein, the mass ratio of BZ and ER was fixed at 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, and the weight fraction of MZ was 3 wt%, 8 wt% and 12 wt% of the content of ER, respectively.
20, and the weight fraction of MZ was 3 wt%, 8 wt% and 12 wt% of the content of ER, respectively.
2.3 Measurement
3. Result and discussion
3.1 Turbidity and morphology
For BZ/ER/MZ blends with different content of MZ, before the curing reaction, all the samples were homogeneous solutions with bright yellow appearance. After curing at 110 °C for 5 h, the transparency of these samples became different: higher content of MZ in the blend, poorer transparency from the appearance (Fig. 1A1–C1). The turbid phenomenon indicated the occurrence of phase separation in BZ/ER/MZ blending system with 8 wt% (Fig. 1B1) and 12 wt% MZ (Fig. 1C1). To further prove the phase separation structures, the fractured surfaces of half cured PBZ/ER/MZ blends were etched with THF and investigated by SEM. As shown in Fig. 1, no phase separation was observed in the sample with 3 wt% MZ (Fig. 1A2), while clearly heterogeneous morphology with irregular spherical domains (0.8–1.2 μm) dispersed in the matrix was observed in the blends with 8 wt% and 12 wt% MZ (Fig. 1B2 and C2).After etching with THF for 24 h, the half cured phase separated PBZ/ER/MZ bulk gradually decomposed into a large amount of white insoluble floccules (Fig. 2 inset). This phenomenon implies that the dispersed phase cannot dissolve in THF, while the matrix (continuous phase) is soluble in THF at the phase separation point. In order to distinguish the component of each rich phase, THF-soluble part and THF-insoluble part were run FTIR and ATR-FTIR scanning separately. Herein, BZ/ER/MZ (8 wt%) blend was selected as an example, and the FTIR spectrum of original BZ/ER/MZ (8 wt%) blend was shown in Fig. 2a for comparison.
 | ||
| Fig. 2 FTIR spectra of BZ/ER/MZ (8 wt%) blend (a), and the THF-soluble part (b) and THF-insoluble part (c) of PBZ/ER/MZ blend after curing at 110 °C for 5 h and etching with THF for 24 h. | ||
As shown in Fig. 2a, the characteristic absorption bands of ER resin (912 cm−1, epoxy group) and BZ resin (943 cm−1, oxazine ring) were both observed in the FTIR spectrum. Meanwhile, the absorption bands of BZ resin at 1035 cm−1 (νs,C–O–C), 1228 cm−1 (νas,C–O–C), 1113 cm−1 (νas,C–N–C) and 1489 cm−1 (1,2-disubstituted benzene) were also observed, which covered the characteristic absorption bands of ER resin at 1041 cm−1 (νs,C–O–C) and 1236 cm−1 (νas,C–O–C). In Fig. 2b, all of the characteristic peaks of BZ monomer were found, indicating that the main component of THF-soluble part was unpolymerized BZ resin. While in Fig. 2c, the disappearance of the peaks of BZ resin at 943 cm−1 and 1489 cm−1, and the observation of absorption bands of ER resin at 1382 cm−1 and 1361 cm−1 (δC–H of isopropyl structures), 1041 cm−1 and 1236 cm−1 indicated that the main component of THF-insoluble part was ER resin. The absence of characteristic absorption band of epoxy group at 912 cm−1 revealed the complete polymerization of ER resin. Therefore, it could be concluded that the embossed dispersed domains observed in Fig. 1B2 and C2 corresponded to ER-rich phases, and the continuous phase (matrix) corresponded to BZ-rich phases.
3.2 The curing reaction of BZ/ER/MZ
The curing reactions of BZ, ER and BZ/ER blend with or without MZ were investigated by DSC measurements. From Fig. 3a and b, it is clearly that MZ can catalyze the polymerization of both ER and BZ resin, but the catalytic effect for ER resin was more obvious. For BZ/ER blend, only one exothermic peak at 244 °C was observed, while under the catalysis of MZ (8 wt%), multi-peaks were observed and the peak temperatures were shifted toward low temperatures (Fig. 3c). Compared with the DSC curves of ER/MZ and BZ/MZ blend, we deduced that in ternary blend the peak at low temperature (151 °C) corresponded to the polymerization of ER with MZ, and the peak at high temperature (197 °C) corresponded to the polymerization of BZ resin. This deduction was consistent with our previous reports.473.3 Polymerization sequence
As shown in Fig. 4, increasing the content of MZ, the exothermic peaks of BZ/ER/MZ blends shifted to low temperatures and the two peaks corresponding to the polymerization of ER and BZ resin were well separated. To further investigate the effect of MZ content on the polymerization of BZ and ER in ternary blending systems, the exothermic curves of BZ/ER/MZ blending systems were well fitted by four Gaussian functions.49 Here, BZ/ER/MZ (8 wt%) blend was shown as an example to study the peak assignment (Fig. 5). According to the above DSC results, Peak 1 and 2 should correspond to the polymerization of ER resin, and Peak 3 and 4 should correspond to the polymerization of BZ resin. Therefore, the reactivity difference between ER and BZ in BZ/ER/MZ blends was mainly dependent on the reactivity difference between the two adjacent peaks (Peak 2 and Peak 3). | ||
| Fig. 5 Peak fit results of the curing exothermal curves of BZ/ER/MZ (8 wt%) blend at the rate of 10 °C min−1. | ||
In this study, the reactivity of ER and BZ resin in BZ/ER/MZ blending system was estimated by activation energy (Eα). To get the Eα information of the blending system, DSC measurements of a series of blends under different heating rates were carried out. The peak fitted temperatures corresponding to Peak2 and Peak3 were recorded and summarized in Table S1.† According to the Ozawa equation:50,51
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β vs. 1/Tp (Fig. S1†) and summarized in Table 1.
β vs. 1/Tp (Fig. S1†) and summarized in Table 1.
As shown in Table 1, when the content of MZ increased from 3 wt% to 12 wt%, the Eα of ER (Eα2) decreased from 78.1 to 67.2 kJ mol−1, while the Eα of BZ (Eα3) almost did not change (105.2–102.7 kJ mol−1). That's to say, increasing the MZ content to some extent could improve the reactivity of ER, while had little effect on that of BZ resin, thus enlarged the reactivity difference ΔEα between ER and BZ (from 34.7% to 52.8%). This result might be due to the fact that most of MZ has been consumed during the polymerization of ER/MZ, only small amount of MZ was left to catalytic the polymerization of BZ resin.
3.4 The growth of molecular chain during phase separation process
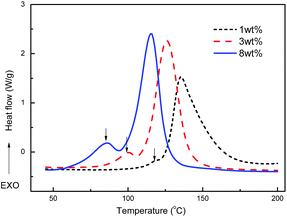
Fig. 6A compared with the DSC curing exothermal curves of BZ/ER/MZ (8 wt%) blending system before and after curing at 110 °C for 5 h. It was clear that, after curing at 110 °C for 5 h, the peak at 151 °C corresponding to the polymerization of ER with MZ disappeared, while the peak at 197 °C corresponding to the polymerization of BZ resin almost had no change. Combining with the conversion–time curves of BZ/ER and ER/MZ systems (Fig. 6B), which showed that the conversion of BZ/MZ was less than 5% after curing at 110 °C for 30 min, while that of ER/MZ could rapidly reach to 80%, we concluded that the polymerization of BZ/ER/MZ blend at 110 °C was mainly associated with the polymerization of ER with MZ. In this case, the polymerization of ER with MZ played an important role for the phase separation of BZ/ER/MZ blends, for clear phase separation was observed after curing at 110 °C for 5 h.DSC curves of ER/MZ blends shown in Fig. 7 manifested that ER has two exothermal peaks under the catalysis of MZ. Increasing the MZ content, both of the two peaks shifted towards low temperatures, and the peak area at low temperature increased. Heise52,53 and Vogt54 et al. studied the polymerization of phenyl glycidyl ether (PGE) with 1,3-unsubstituted MZ and attributed the peak at the low temperature to the MZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PGE (1
PGE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) adduct formation (Scheme 2, step 1) and the main peak to the alkoxide-initiated polymerization (Scheme 2, step 2). Farkas and Strohm55 proposed that MZ
2) adduct formation (Scheme 2, step 1) and the main peak to the alkoxide-initiated polymerization (Scheme 2, step 2). Farkas and Strohm55 proposed that MZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PGE (1
PGE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) adducts were assumed to act as the initiators for the polymerization of PGE by an etherification reaction in which the reactive alkoxide anion was the propagating species. Meanwhile, Barton et al. claimed that the adduct formation step and alkoxide-initiated polymerization of ER/MZ occurred step by step, but not simultaneously.56 According to the reported polymerization mechanism of ER/MZ shown in Scheme 2, the adduct formation step tends to form a linear polymer, and the subsequent anionic polymerization causes the crosslinking reaction and leads to the formation of three dimensional network structure.26 In this case, increasing the MZ content would form longer ER–MZ oligomers firstly, and then resulted in a looser network structures.
2) adducts were assumed to act as the initiators for the polymerization of PGE by an etherification reaction in which the reactive alkoxide anion was the propagating species. Meanwhile, Barton et al. claimed that the adduct formation step and alkoxide-initiated polymerization of ER/MZ occurred step by step, but not simultaneously.56 According to the reported polymerization mechanism of ER/MZ shown in Scheme 2, the adduct formation step tends to form a linear polymer, and the subsequent anionic polymerization causes the crosslinking reaction and leads to the formation of three dimensional network structure.26 In this case, increasing the MZ content would form longer ER–MZ oligomers firstly, and then resulted in a looser network structures.
Fig. 8 shows the storage modulus and tan delta curves of cured ER/MZ blends. The storage modulus (Er) at the rubber plateau decreased with the increase of MZ content. According to the theory of rubber elasticity, the crosslinking density (v) of effective network chains and the molecular weight between crosslinks (Mc) can be estimated from Er as13
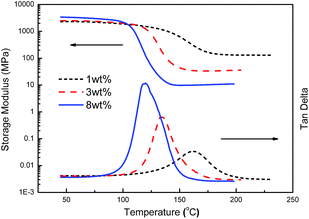 | ||
| Fig. 8 Storage modulus and tan delta curves of ER/MZ blend with different content of MZ after curing at 110 °C/20 h + 180 °C/2 h. | ||
As shown in Table 2, when the content of MZ increased from 1 wt% to 8 wt%, the crosslinking density of ER/MZ blends decreased from 11.6 × 103 to 0.9 × 103 mol m−3, and the molecular weight between crosslinks increased 12 times. This result confirmed that with high MZ content, the polymerization of ER was tend to form long linear ER polymer chains before the gelation, and then resulted in a looser network. Otherwise, the polymerization of ER was tend to form short polymer chains before the gelation, and resulted in a tight network.
3.5 Rheological behavior
The real-time isothermal (110 °C) rheological results for BZ/ER/MZ blending systems with different MZ content was measured and shown in Fig. 9. As the gel point for thermosetting resin can be estimated from the crossover point of storage modulus (G′, elastic response) and loss modulus (G′′, viscous behavior),57,58 it is clear that the gel time of BZ/ER/MZ blends decreased from 2646 s to 1906 s, and the viscosity at gel point increased 14 times as the content of MZ increased from 3 wt% to 8 wt%. While further increasing the MZ content to 12 wt%, no gel point was observed (G′ > G′′ during the whole curing process), but high viscosity was obtained in a very short time (<1500 s). What's more, the information about the increasing rate and the final viscosity of BZ/ER/MZ blends also were obtained from Fig. 9B. For maximum content of MZ (c), the system viscosity had a rapid enhancement in a short time (t < 1500 s) and then balanced at 4969 Pa s. For minimum content of MZ (a), the system viscosity increased very slowly at the initial stage (t < 2000 s), but underwent a rapid enhancement around the gel point and the final viscosity (76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 082 Pa s) was 15 times higher than that of curve (c). As for the middle content of MZ (b), the above situation fell in between (a) and (c).
082 Pa s) was 15 times higher than that of curve (c). As for the middle content of MZ (b), the above situation fell in between (a) and (c).
From the above analysis, it was interesting to note that when the MZ content was high to 12 wt%, no gelation phenomenon was observed. And increasing the content of MZ led to a fast increase of the viscosity and high viscosity before the gelation but a relatively lower viscosity at balanced domains. Because the curing reaction of BZ/ER/MZ blend at 110 °C was mainly associated with the polymerization of ER with MZ, the gelation and isothermal viscosity various both contributed to the polymerization of ER/MZ. In our systems, when the MZ content was 12 wt%, the molar ratio of epoxy group of ER resin to the N function groups of MZ compound was 2.5/2.0. Most of the epoxy groups was consumed during the adduct formation process, and only few of them was left to take part in the crosslink reaction (alkoxide-initiated polymerization). That's why no gelation phenomenon could be observed in BZ/ER/MZ blend with 12 wt% of MZ. This result indirectly proved that looser or linear/branched poly-ER would be obtained with the increase of MZ content, which was consistent with the results shown in Table 2. What's more, before the gelation, the fast increase of viscosity and the high viscosity value indicated the fast growth of the ER molecular chains and the formation of high molecular weight of linear ER–MZ oligomers, which decreased the system entropy of mixing, and increased the dynamic asymmetric between ER and BZ, thus favoring the occurrence of phase separation.15 Meanwhile, the relative low complex viscosity at balanced domains indicated the formation of branched or looser poly-ER network structures, which could allow the free diffusion of BZ monomers in and out of the ER network. Therefore, high MZ content was the key to balance the opposite influences of curing reaction of ER resin on the phase separation of BZ/ER/MZ blending system.
3.6 Phase separation process
According to the results and discussions given in the previous sections, the relationships among MZ content, curing reaction, rheological behavior and the phase separation of BZ/ER/MZ blending system were obtained as illustrated in Scheme 3. | ||
| Scheme 3 Schematic of the relationship among MZ content, curing reaction and phase separation of BZ/ER/MZ blends at 110 °C. | ||
Before the curing reaction, BZ resin, ER resin, and MZ were homogeneously mixed. When the reaction started at 110 °C, ER resin could homopolymerize preferentially under the catalyst of MZ to form linear ER–MZ oligomers and then to form branched or crosslinked ER networks. But the ER network structures and the final morphology of PBZ/ER/MZ blends were depended on the MZ content added in the system:
(A) If the MZ content was low (≤3 wt%), the molecular chain of ER–MZ oligomer formed in the first step shown in Scheme 2 was much shorter, which led to the formation of much tighter ER-network in the following alkoxy-anion-initiated polymerization of ER. In this case, at the beginning, the slowly polymerization of ER/MZ neither enlarged the dynamic asymmetry between BZ and ER, nor effectively reduced the systemic entropy. And in the later stage, the formation of the highly crosslinked ER networks led to the increase of viscosity and the reduction of the mobility of ER network, thus inhibited the separation of ER network from the BZ matrix. Consequently, during the whole polymerization process, phase separation was impossible and only homogeneous structure was obtained.
(B) If the MZ content was high enough (≥8 wt%), the polymerization rate of ER was fast, and the molecular chain of ER–MZ oligomer was much longer. In this case, the dynamic asymmetry between ER-network and BZ resin was enlarged, and the system entropy was reduced sharply. Both of those led to the decrease of the compatibility of the ER polymer with surrounding BZ resin. In the late stage, the formation of branched or looser ER-network led to a relative low systemic viscosity, which allowed the free diffusion of BZ resin out from ER network. During this process, lightly crosslinked ER network gradually shrunk to exclude the entrapped BZ molecules due to its viscoelastic property, and finally micro-sized dispersed ER-rich phases were obtained.
4. Conclusions
In BZ/ER/MZ blending systems, the curing reaction of BZ/ER/MZ blend at 110 °C was mainly associated with the polymerization of ER with MZ. Increasing the MZ content could accelerate the polymerization of ER and enlarge the reactivity difference (ΔEα) between ER and BZ resin, therefore reduced the possibility of copolymerization between BZ and ER. What's more, increase the MZ content led to the linear growth of the ER molecular chains and resulted in a looser network structures, which could not only increase the dynamic asymmetry between BZ and ER, but also retain the system viscosity in a relative low range, therefore was the key to prepare multiphase structures in BZ/ER/MZ blend. That's why 0.8–1.2 μm sized sea-island phase structures were observed in BZ/ER/MZ blending with 8 wt% and 12 wt% of MZ systems but not in with 3 wt% of MZ system. The finial mechanical properties, especially the toughness of the BZ/ER/MZ blends will be systematically and deeply investigated in the future studies.Acknowledgements
Supports for this project were provided by the National Natural Science Foundation of China (Project no. 50873062) and Doctoral Fund of Ministry of Education of China (Project no. 20090181110030).Notes and references
- J. P. Pascault, H. Sautereau, J. Verdu and R. J. J. Williams, Thermosetting polymers, Marcel Dekker, New York, 2002, ch. 1, pp. 12–15 Search PubMed.
- A. Gardziella, L. A. Pilato and A. Knop, Phenolic Resins: Chemistry, Applications, Standardization, Safety and Ecology, Springer, Berlin, 2nd edn, 2000, ch. 6 Search PubMed.
- J. B. Cho, J. W. Hwang, K. Cho, J. H. An and C. E. Park, Polymer, 1993, 34, 4832 CrossRef CAS.
- D. Klempner, L. H. Sperling and L. A. Utracki, Interpenetrating Polymer Networks, American Chemical Society, Washington, 1st edn, 1994, ch. 1 Search PubMed.
- J. P. Pascault and R. J. J. Williams, in Polymer blends, Vol. 1: Formulation, ed. D. R. Paul and C. B. Bucknall, Wiley, New York, 2000, ch. 13, pp. 379–415 Search PubMed.
- S. Thomas, A. Boudenne, L. Ibos and Y. Candau, in Handbook of multiphase polymer systems, ed. A. Boudenne, L. Ibos, Y. Candau and S. Thomas, Wiley, United Kingdom, 1st edn, 2011, vol. 1, pp. 1–12 Search PubMed.
- R. J. J. Williams, B. A. Rozenberg and J. P. Pascault, Adv. Polym. Sci., 1997, 128, 95 CrossRef CAS.
- Y. S. Kim and S. C. Kim, Macromolecules, 1999, 32, 2334 CrossRef CAS.
- H. Kim and K. Char, Ind. Eng. Chem. Res., 2000, 39, 955 CrossRef CAS.
- Z. Wang, Q. Ran, R. Zhu and Y. Gu, RSC Adv., 2013, 3, 1350 RSC.
- W. J. Gan, Y. F. Yu, M. H. Wang, Q. S. Tao and S. J. Li, Macromolecules, 2003, 36, 7746 CrossRef CAS.
- H. Lü and S. Zheng, Polymer, 2003, 44, 4689 CrossRef.
- H. Ishida and D. J. Allen, Polymer, 1996, 37, 4487 CrossRef CAS.
- J. Yang, M. A. Winnik, D. Ylitalo and R. J. DeVoe, Macromolecules, 1996, 29, 7047 CrossRef CAS.
- K. Dean and W. D. Cook, Macromolecules, 2002, 35, 7942 CrossRef CAS.
- Y. S. Yang and L. J. Lee, Macromolecules, 1987, 20, 1490 CrossRef CAS.
- C. Jubsilp and S. Rimdusit, in Handbook of benzoxazine resins, ed. H. Ishida and T. Agag, Elsevier, Amsterdam, 1st edn, 2011, ch. 7, pp. 169–172 Search PubMed.
- S. Rimdusit and H. Ishida, Polymer, 2000, 41, 7941 CrossRef CAS.
- S. Rimdusit and H. Ishida, J. Polym. Sci., Part B: Polym. Phys., 2000, 38, 1687 CrossRef CAS.
- M. A. Espinosa, V. Cadiz and M. Galia, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 279 CrossRef CAS.
- N. N. Ghosh, B. Kiskan and Y. Yagci, Prog. Polym. Sci., 2007, 32, 1344–1391 CrossRef CAS PubMed.
- R. Jain, A. K. Narula and V. Choudhary, J. Appl. Polym. Sci., 2007, 106, 3327 CrossRef CAS.
- S. Mortimer, A. J. Ryan and J. L. Stanford, Macromolecules, 2001, 34, 2973 CrossRef CAS.
- I. Glavchev, K. Petrova and I. Devedjiev, Polym. Test., 2002, 21, 89 CrossRef CAS.
- M. Ghaemy and S. Sadiady, Iran. Polym. J., 2006, 15, 103 CAS.
- Y. R. Ham, S. H. Kim, Y. J. Shin, D. H. Lee, M. Yang, J. H. Min and J. S. Shin, J. Ind. Eng. Chem., 2010, 16, 556 CrossRef CAS PubMed.
- S. K. Ooi, W. D. Cook, G. P. Simon and C. H. Such, Polymer, 2000, 41, 3639 CrossRef CAS.
- L. S. Xu and J. R. Schlup, J. Appl. Polym. Sci., 1998, 67, 895 CrossRef CAS.
- B. Bilyeu, W. Brostow and K. Menard, US Pat., 7 868 067 B2, 2011.
- B. N. Burns, H. Woolfson, P. Malone and J. Wigham, US Pat., 6 872 762 B2, 2005.
- Y. X. Wang and H. Ishida, Polymer, 1999, 40, 4563 CrossRef CAS.
- S. Atsushi, K. Ryoichi, N. Hiroshi, A. Kazuya and E. Takeshi, Macromolecules, 2008, 41, 9030 CrossRef.
- T. Agag, C. R. Arza, F. H. J. Maurer and H. Ishida, Macromolecules, 2012, 43, 2748 CrossRef.
- S. Grishchuk, Z. Mbhele, S. Schmitt and J. Karger-Kocsis, eXPRESS Polym. Lett., 2011, 5, 273 CrossRef CAS.
- H. Ishida and Y. Rodriguez, Polymer, 1995, 36, 3151 CrossRef CAS.
- J. Dunkers and H. Ishida, J. Polym. Sci., Part A: Polym. Chem., 1999, 37, 1913 CrossRef CAS.
- K. Naoki and M. Schozo, JP Pat., 191775, 2000.
- B. S. Rao, K. R. Reddy, S. K. Pathak and A. R. Pasala, Polym. Int., 2005, 54, 1371 CrossRef CAS.
- S. Rimdusit, S. Pirstpindvong, W. Tanthapanichakoon and S. Damrongsakkul, Polym. Eng. Sci., 2005, 45, 288 CAS.
- H. Kimura, Y. Murata, A. Matsumoto, K. Hasegawa, K. Ohtsuka and A. Fukuda, J. Appl. Polym. Sci., 1999, 74, 2266 CrossRef CAS.
- H. Kimura, A. Matsumoto and K. Ohtsuka, J. Appl. Polym. Sci., 2008, 109, 1248 CrossRef CAS.
- H. Kimura, A. Matsumoto and K. Ohtsuka, J. Appl. Polym. Sci., 2009, 112, 1762 CrossRef CAS.
- B. S. Rao and S. K. Pathak, J. Appl. Polym. Sci., 2006, 100, 3956 CrossRef CAS.
- S. Grishchuk, S. Schmitt, O. C. Vorster and J. Karger-Kocsis, J. Appl. Polym. Sci., 2012, 124, 2824 CrossRef CAS.
- P. Zhao, Q. Zhou, X. Liu, R. Zhu, Q. Ran and Y. Gu, Polym. J., 2013, 45, 637 CrossRef CAS.
- H. Wang, P. Zhao, H. Ling, Q. Ran and Y. Gu, J. Appl. Polym. Sci., 2013, 127, 2169 CrossRef CAS.
- P. Zhao, Q. Zhou, Y. Deng, R. Zhu and Y. Gu, RSC Adv., 2014, 4, 238 RSC.
- H. Xiang, H. Ling, J. Wang, L. Song and Y. Gu, Polym. Compos., 2005, 26, 563 CrossRef CAS.
- C. Jubsilp, S. Damrongsakkul, T. Takeichi and S. Rimdusit, Thermochim. Acta, 2006, 447, 131 CrossRef CAS PubMed.
- R. Serra, J. Sempere and R. Nomen, Thermochim. Acta, 1998, 316, 37 CrossRef CAS.
- A. K. Higham, L. A. Garber, D. C. Latshaw, C. K. Hall, J. A. Pojman and S. A. Khan, Macromolecules, 2014, 47, 821 CrossRef CAS.
- M. S. Heise and G. C. Martin, J. Appl. Polym. Sci., 1990, 39, 721 CrossRef CAS.
- M. S. Heise and G. C. Martin, Macromolecules, 1989, 22, 99 CrossRef CAS.
- J. Vogt, J. Adhes., 1987, 22, 139 CrossRef CAS.
- A. Farkas and P. F. Strohm, J. Appl. Polym. Sci., 1968, 12, 159 CrossRef CAS.
- J. M. Barton and P. M. Shepherd, Die Makromolekulare Chemie, 1975, 176, 919 CrossRef CAS.
- L. Weng, M. Chen and W. Chen, Biomacromolecules, 2007, 8, 1109 CrossRef CAS PubMed.
- T. A. Ozawa, Bull. Chem. Soc. Jpn., 1965, 38, 1881 CrossRef CAS.
Footnote |
† Electronic supplementary information (ESI) available: The fitted peak temperature (Table S1) and the Ozawa plots of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β vs. 1/T for fitted Peak2 and Peak3 (Fig. S1) of BZ/ER/MZ blends. See DOI: 10.1039/c4ra10484f β vs. 1/T for fitted Peak2 and Peak3 (Fig. S1) of BZ/ER/MZ blends. See DOI: 10.1039/c4ra10484f |
| This journal is © The Royal Society of Chemistry 2014 |