Fabrication of porous TiO2–SiO2 multifunctional anti-reflection coatings by sol–gel spin coating method†
Abstract
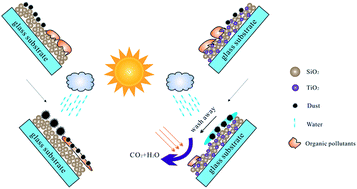
In this study, we report that an antireflective coating with multifunctional properties, including high transparency, self-cleaning and abrasion resistance, was prepared by a facile sol–gel spin-coating method. The average transmittance of single coated glass substrates was greater than 93% in the wavelength range of 300–1000 nm compared to 90.1% for bare glass substrate. The photocatalytic activity of the coatings showed a close dependence on the content of TiO2 nanoparticles. The coatings showed the ability to decompose air borne organic contaminants (gaseous benzene) and applied contaminants (fingerprints) under UV irradiation when the content of TiO2 was greater than 10%. After illumination by UV light, the TiO2–SiO2 coatings showed hydrophilic properties with a water contact angle of about 1°, which favored greatly the self-cleaning function of the coatings. When the content of TiO2 was 10%, the critical load of the coating increased up to 305 μN as compared to that of the base-catalyzed SiO2 coating (198 μN), which indicated the abrasion resistance property of this coating. This approach suggested novel opportunities to a variety of practical applications, including windows of high rise buildings, solar thermal collectors and solar cell cover glasses, etc.

 Please wait while we load your content...
Please wait while we load your content...