Enhanced photoelectroctatlytic performance of etched 3C–SiC thin film for water splitting under visible light
Abstract
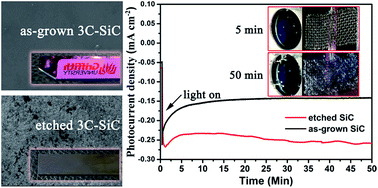
3C–SiC films have robust mechanical and physicochemical properties and a narrow band gap (2.36 eV). In this work, a robust p-type 3C–SiC thin film is grown on a large silicon substrate using a low temperature alternating supply epitaxy method. The film is heavily doped with Al in order to achieve high conductivity and allow photoelectrocatalytic splitting of water for hydrogen production under visible light. The as-grown thin film is further treated with a facile dry etching process in order to improve the surface area and induce a light trap structure. In comparison with the as-grown sample, the etched thin film possesses substantially improved photoelectrocatalytic performance due to increased light absorption, larger surface area and reduced recombination rate of photoelectron and holes.

 Please wait while we load your content...
Please wait while we load your content...