Sonochemical synthesis of highly photoluminescent carbon nanodots†
Abstract
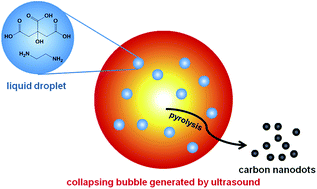
We present a convenient sonochemical approach for the synthesis of highly photoluminescent carbon nanodots (CDs). CDs were synthesized via pyrolysis of carbon precursors inside implosively collapsing bubbles. We further demonstrate that these CDs can be used for in vitro bioimaging.

 Please wait while we load your content...
Please wait while we load your content...