Preparation of Sr8Mg1−mZnmY(PO4)7:Eu2+ solid solutions and their luminescence properties
Wanjun Tang* and
Huaijia Xue
Hubei Key Laboratory for Catalysis and Material Science, College of Chemistry and Material Science, South-Central University for Nationalities, Wuhan 430074, P.R. China. E-mail: tangmailbox@126.com; Fax: +86-27-67842752; Tel: +86-27-67842752
First published on 11th November 2014
Abstract
A series of orthophosphate solid solutions with a general formula of Sr8Mg1−mZnmY(PO4)7: Eu2+ (0 ≤ m ≤ 1) were synthesized by the combustion-assisted synthesis technique. The XRD patterns confirm the formation of a solid solution of Sr8Mg1−mZnmY(PO4)7 and lattice parameters of the solid solutions are linearly dependent on m. The excitation spectra of these phosphors show broad band excitation and absorption in the 250–470 nm near ultraviolet (UV) region, which is ascribed to the 4f7–4f65d1 transitions of Eu2+. With an increase in m, the emission color shifts from yellow to green under near-UV excitation, which is rationalized in terms of two competing factors of the crystal filed strength and bond covalence. All the results indicate that the solid solution Sr8Mg1−mZnmY(PO4)7: Eu2+ can be a good candidate of phosphor applicable to near-UV LEDs for solid-state lighting.
1. Introduction
Light-emitting diodes (LEDs) are considered as a promising technology for next generation solid-state lighting systems because of their high efficiency, small size, compactness, safety, high material stability, durability, and resultant energy-saving capacity.1 White LEDs fabricated with multi-color phosphors and near ultraviolet (n-UV) LEDs are considered to be a very promising route to realize a high-quality light source.2 For LED phosphors, they are generally required to have high conversion efficiency, excellent chemical stability and high thermal quenching temperature. In addition, they are also expected to show a tunable ability in emission color, allowing the white LED designers to have greater flexibility in choosing emission wavelength for the improvement either in correlated color temperature or color rendering index of white LEDs for the phosphor applications.3 Therefore, it has a great impact on research on luminescent materials (phosphors).Eu2+ doped phosphors have been widely investigated in the past years because of their promising applications in LEDs. The divalent rare-earth Eu2+ ion, which has 4f7 ground state and the excited state of 4f65d, usually give broad emission due to parity allowed 4f7–4f65d1 transitions.4 The peak positions in the emission spectra depend strongly on the nature of the Eu2+ surroundings. Therefore Eu2+ can be efficiently excited in a broad spectral range depending on the host lattices in which it is incorporated. The absorption and emission bands of activators Eu2+ can also be well controlled by varying the crystal field or the bond covalence depending on site size, site symmetry and coordination environment of activator ions. In order to realize desirable luminescent properties, one promising way is to modify the composition of the host lattice through a solid solution with similar crystal structure.5
Phosphate compounds are a kind of important luminescence host, which can produce plenty of crystal field environments imposed on emission centers. Recently, a variety of phosphates with the formula Sr8MgRe(PO4)7 have been reported in the literature, such as Sr8MgSc(PO4)7,6 Sr8MgLu(PO4)7,7 Sr8MgGd(PO4)7,8 and Sr8MgLa(PO4)7.9 Sr8MgRe(PO4)7 hosts are good candidates as host structures due to several merits, such as high chemical stability and good thermal quenching, and exhibit intense luminescence for white LEDs application when activated with Eu2+. Additionally, the variety of the structures in these compounds makes it possible to finely tune the physical/chemical properties to design new materials. Recently, Huang et al. reported9 that the Eu2+-activated Sr8MgY(PO4)7 exhibited a broad yellow emission upon excitation at near ultraviolet (near-UV) light and a warm white-light near-UV LED was fabricated using a near-UV 400 nm chip pumped by a phosphor blend of blue-emitting BaMgAl10O17: Eu2+ and yellow-emitting Sr8MgY(PO4)7: Eu2+.
In this paper, we present a strategy to tune the emission color of Sr8MgY(PO4)7: Eu2+ phosphor by introducing foreign cations, Zn2+, to modify the crystal field strength acting on Eu2+ activators. The emitting color can be tuned from yellow to green using this solid solution strategy. The modification of crystal field is discussed in details in connection with crystal structure changes and photoluminescence excitation. The solid solution strategy suggested here greatly improves certain key properties of these promising phosphate phosphors.
2. Experimental
2.1 Materials and methods
In this study, polycrystalline phosphors with the compositions of (Sr0.99Eu0.01)8(Mg1−mZnm)Y(PO4)7 (hereafter abbreviated as Znm-SMYP: Eu2+, 0 ≤ m ≤ 1) were synthesized by a combustion-assisted synthesis method.10 Stoichiometric amount of raw materials NH4H2PO4 (analytical reagent, AR), Mg(NO3)2·6H2O (AR), Sr(NO3)2 (AR), and Zn(NO3)2·6H2O (AR) were thoroughly mixed, and an appropriate amount of CO(NH2)2 (AR) was added as fuel. Y2O3 (99.99%) and Eu2O3 (99.99%) were dissolved in HNO3 to convert into the corresponding nitrate completely. These reagents were dissolved in water and introduced into a muffle furnace maintained at 600 °C for 5 min. The obtained precursors were fired at 1300 °C for 3 h under a 5% H2/95% N2 mixture gas to form crystalline phosphates.2.2 Characterization
The phase purity of the as-prepared samples was checked using a Bruker D8 diffractometer (Bruker Co., Karlsruhe, Germany) in the 2θ range from 10° to 80°, with graphite monochromatized Cu Kα radiation (λ = 0.15405 nm) operating at 40 kV and 40 mA. The lattice constants were calculated by cell refinement fitting with MDI Jade 5.0 on the basis of the XRD data. The excitation (PLE) and emission (PL) spectra were recorded on a Jasco FP-6500 fluorescence spectrophotometer (Jasco Co., Tokyo, Japan) equipped with a 150 W xenon lamp as the excitation light source. Scanning electron micrographs (SEM) were taken on a Hitachi SU8000 scanning electron microscopy (Hitachi, Japan). The chemical composition of the particles was carried out using the SEM equipped with energy dispersive X-ray spectroscopy (EDX). For comparison, all measurements were performed at room temperature with the identical instrumental parameters.3. Results and discussion
3.1 Phase identification and crystal structure
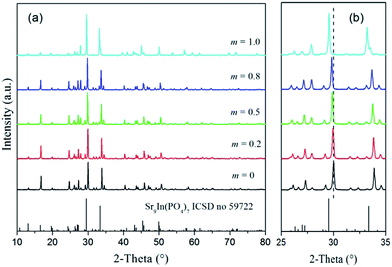
The crystal structure studies of Sr8MgY(PO4)7 have been carried out by Huang et al., which reported that the compound Sr8MgY(PO4)7 is isostructural with Sr9In(PO4)7. In the structure of Sr8MgY(PO4)7, the Sr2+ ions have five different coordination environments: Sr(1) is defined as being eight-coordinated; Sr(2), Sr(3), and Sr(4) are defined as being nine-coordinated; and Sr(5) is defined as being ten-coordinated. The crystal structures of Sr8Mg1−mZnmY(PO4)7 were evolved from the Sr8MgY(PO4)7 phase by the iso-structural replacement of Mg/Zn atoms. Fig. 1 shows the XRD patterns of the synthesized Sr8Mg1−mZnmY(PO4)7 powders at various concentrations (0 ≤ m ≤ 1) of Zn2+. No peaks of the raw materials or other allotropic forms are detected up to m = 1. The Sr8MgY(PO4)7 (m = 0, hereafter abbreviated as SMYP) powders show the characteristic pattern of the monoclinic structure (space group I2/a), matches well with Inorganic Crystal Structure Database (ICSD) file no. 59722. All the diffraction peaks of the prepared sample SMYP agrees well with those reported previously.9 There is no obvious difference among these XRD patterns when 0 ≤ m ≤ 1, which are all consistent with ICSD file no. 59722. There is no alien diffraction peaks. Thus the samples with 0 ≤ m ≤ 1 can be indexed to single monoclinic phase with I2/a space group. This indicates that doping of Zn2+ does not significantly change the phase structure of samples. However, the diffraction peaks shifts slightly to the lower angle side with concentration increase of Zn2+ ions, as shown in Fig. 1b. This observation indicates that substitution of larger Zn2+ (r = 0.90 Å, CN = 8) for Mg2+ (r = 0.89 Å, CN = 8) causes the changes in the lattice constant of host lattice, which can be calculated by indexing XRD data. Powder XRD analyses were performed to determine the chemical purity and phase homogeneity of the Sr8Mg1−mZnmY(PO4)7 powders. The lattice parameters were calculated based on the experimental XRD profiles using the cell refinement software. The structural parameters reported on Sr9In(PO4)7 were used as initial parameters in the Rietveld analysis. Structural refinements of Sr8Mg1−mZnmY(PO4)7 were all performed by a monoclinic space group of I2/a. The refined unit cell parameters for m = 0 and 1 are summarized in Table 1. It is noticeable that in the ICSD there are no powder diffraction data or reference data that could be found for the end compound of Sr8ZnY(PO4)7 (hereafter abbreviated as SZYP, m = 1). The results indicate that the as-prepared SZYP sample is also obtained as a single phase compound that matches well with ICSD file no. 59722.| Formula | Sr9In(PO4)7 | Sr8MgY(PO4)7 | Sr8ZnY(PO4)7 |
|---|---|---|---|
| a (Å) | 18.0425(2) | 18.47387(5) | 18.4903(2) |
| b (Å) | 10.66307(4) | 10.10571(5) | 10.07955(1) |
| c (Å) | 18.3714(2) | 18.14809(3) | 18.32116(3) |
| β (°) | 132.9263(5) | 131.9509(2) | 132.17(1) |
| V (Å3) | 2588.02 | 2519.79 | 2530.71 |
| Z | 4 | 4 | 4 |
As seen in Fig. 1 (right part), by substituting Mg2+ with Zn2+ ions, the diffraction peaks of these samples shift slightly towards the lower 2θ angle. This can be attributed to the lattice expansion due to the substitution of Mg2+ by the larger ions of Zn2+. The change of lattice constant of host lattice can be calculated by indexing XRD data. As shown in Fig. 2, lattice constant c increase linearly as Zn2+ concentration increases, while the lattice constants a and b have little changes. This observation suggests that introduction of Zn2+ enlarges and distorts the SMYP lattice. The distortion of host lattice will have a strong impact on the luminescence properties of Eu2+ ions with 5d energy levels, which will be discussed in section of photoluminescence. The ionic radii of the eight- and nine-coordinated Sr2+ ions are 1.26 and 1.31 Å, respectively. Meanwhile, the ionic radii of the eight- and nine-coordinated Eu2+ ions are 1.25 and 1.3 Å, respectively. On account of the matching of ionic radii, the Eu2+ ions are expected to randomly occupy the Sr2+ or Mg2+ or Zn2+ or Y3+ ions sites in the Znm-SMYP host.
In our previous study,11 the crystal structure of Sr8(Mg,Zn)La(PO4)7 was investigated by changing the content of Zn substitution. Although the crystal structures of Sr8(Mg,Zn)La(PO4)7 were evolved from the Sr8MgLa(PO4)7 phase by the iso-structural replacement of Mg/Zn atoms, Sr8(Mg,Zn)La(PO4)7 formed a eutectic mixture containing two distinct phases, Sr8MgLa(PO4)7 and Sr8ZnLa(PO4)7. On the contrary, the XRD patterns of Znm-SMYP in Fig. 1 show no obvious difference among the five XRD patterns when 0 ≤ m ≤ 1. There is no alien diffraction peaks. Thus the samples with 0 ≤ m ≤ 1 can be indexed to single monoclinic phase. This indicates that doping of Zn2+ does not significantly change the phase structure of samples. Fig. 2 displays the variation of the lattice parameters with m. The lattice constants either linearly increase of decrease with increasing m, indicating Vegard's law is followed,12 proving complete solid solution between the two distinct end-member compounds, SMYP and SZYP.
In order to investigate the precursor, a series of other measurements were performed on the samples. The elements detected by EDX are largely comprised of Sr, Mg, Zn, Y, P, and O. The calculated value of stoichiometry from the EDX data was found to be consistent with the composition of the precursor used in the experiment and is shown in Table 2. The analyzed Zn to Y ratio in the Sr8(Mg,Zn)Y(PO4)7 phase is appreciably lower compared to that of the reaction system, indicating that a part of Zn was eliminated from the surface of phosphor powders by evaporation during the high temperature post-treatment.
| m | 0 | 0.2 | 0.5 | 0.8 | 1 |
|---|---|---|---|---|---|
| Y | 1 | 1 | 1 | 1 | 1 |
| Sr | 8.06 | 7.81 | 7.65 | 8.15 | 7.91 |
| P | 6.86 | 7.13 | 6.83 | 6.55 | 6.78 |
| Mg | 1.05 | 0.82 | 0.52 | 0.19 | — |
| Zn | — | 0.08 | 0.35 | 0.53 | 0.72 |
3.2 SEM images of Znm-SMYP: Eu2+ phosphor
Fig. 3 shows the representative SEM micrograph of Znm-SMYP: Eu2+, m = 0, 0.5 and 1. The phosphors have an irregular elliptical shape, and the particle sizes of the obtained powders are no more than 100 μm. There was an aggregation of the particles. The microstructural features of the specimens are generally similar except for the slightly different grain sizes. This indicated that the addition of zinc could accelerate the growth of the grains. These particles show a slight tendency to agglomerate, especially for the specimen with the addition of zinc. Though the uniform morphology is not obtained by this route, it is acceptable for the white LED application as the particle size requirement is not as strict as that in the plasma display panel (PDP) field.3.3 Photoluminescence properties
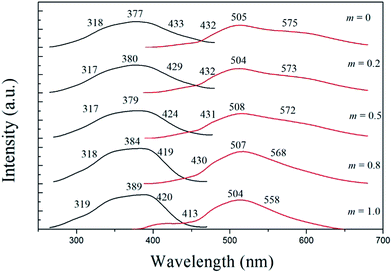
The PL/PLE spectra of SMYP: Eu2+ and the deconvoluted Gaussian components are shown in Fig. 4. The observed PLE spectrum monitoring by 513 nm is composed of one strong absorption peak at 377 nm and two weak absorption peaks located around 318 and 433 nm in the spectral range from 250 to 470 nm, which is attributed to 4f7(8S7/2)–4f65d transitions of the doped Eu2+ ions. The asymmetric shape of the PLE spectrum is due to the complexity of the 5d excited state of Eu2+. The broad band excitation perfectly fulfils the requirements for a conversion phosphor excited by n-UV to blue light. Since phosphate hosts exhibit absorption in the vacuum ultraviolet region (100–200 nm),13 these excitation bands in the near-UV range mainly arise from the 4f65d1 multiplets of Eu2+ excitation states. An asymmetric yellow emitting broad band was observed in the wavelength range of 380–680 nm, which corresponds to the allowed 4f65d1 → 4f7 electronic transitions of Eu2+. The emission spectrum shows the strongest emission around 513 nm and two weak shoulders in the high energy region at 432 nm and in the low energy region at 575 nm, respectively. The asymmetric emission spectrum of SMYP: Eu2+ indicates that the Eu2+ ions have more than one emission centre in the SMYP lattice. In the SMYP crystal structure, an 8f site corresponding to the Ca4 and Ca6 sites in the β-Ca3(PO4)2 type structure is vacant and Sr/Mg has five different coordination numbers: Sr/Mg2 is defined to be eight-coordinated; Sr/Mg1, Sr/Mg3, Sr/Mg4, and Sr/Mg5 are nine-coordinated. Huang et al. reported6 that the PL spectra of the SMYP: Eu2+ phosphor could be decomposed into five Gaussian profiles in the wavelength range of 450–800 nm. However, due to the serious overlap, the PL spectrum of the SMYP: Eu2+ phosphor obtained in this paper can be decomposed into only three Gaussian profiles with peaks centered at 432 nm (Eu1, 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 148 cm−1), 504 nm (Eu2, 19
148 cm−1), 504 nm (Eu2, 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 841 cm−1), and 575 nm (Eu3, 17
841 cm−1), and 575 nm (Eu3, 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 391 cm−1) in the wavelength range of 380–680 nm. These peaks can be ascribed to at least three different emission sites, which could be identified as the different coordination environments of the Sr2+ ions being occupied by Eu2+ ions. No other characteristic emission peaks from Eu3+ are observed in the PL spectrum, indicating that Eu3+ have been reduced to Eu2+ completely in our experiments.
391 cm−1) in the wavelength range of 380–680 nm. These peaks can be ascribed to at least three different emission sites, which could be identified as the different coordination environments of the Sr2+ ions being occupied by Eu2+ ions. No other characteristic emission peaks from Eu3+ are observed in the PL spectrum, indicating that Eu3+ have been reduced to Eu2+ completely in our experiments.
 | ||
| Fig. 4 PLE and PL spectra of SMYP: Eu2+ (a) and SZYP: Eu2+ (b) phosphors. The dashed lines are Gaussian deconvolution curves. | ||
It is well-known that the 5d wave function has large spatial extension, and it depends on the surroundings of Eu2+ ions. Thus, the emission position of the Eu2+ ion is strongly dependent on its local environment. In order to further verify the occupancy ascription of the three emission bands, the well-known experiential equation given by Van Uitert has been used to qualitatively analyse the present experimental result.14 According to the report of Van Uitert, for Eu2+ in suitable matrices, the following experiential equation, provides a good fit to the emission peak and excitation edge data. On the basis of this, the problem about which kind of crystallographic site substituted by Eu2+ in SMYP can be investigated theoretically.
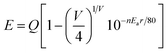 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1); V is the valence of the Eu2+ ion (V = 2); n is the number of anions in the immediate shell around the Eu2+ ion; r is the effective radius of the host cation replaced by the Eu2+ ion (in Å); and “Ea” is the electron affinity of the anion atoms (in eV) dependent on the anion complex type. Here, Ea is approximately determined as 2.19 eV for the phosphate host.15 Table 3 show the experimental and calculated emission wavelengths of Eu2+ ions occupying different cation ions and coordination environments in the SMYP host. The result indicate that the experimental values agree well with the calculated values and the broad asymmetric yellow-emitting band is due to Eu2+ ions occupying eight-coordinated Sr2+ and Mg2+, and six-coordinated Y3+ site. Therefore, we can draw a conclusion that the first band centered at 432 nm (Eu1) is attributed to the 5d–4f transitions of Eu2+ occupying Sr2+ and Mg2+ sites with nine-coordination, and the second band centered at 504 nm is due to the 5d–4f transitions of Eu2+ occupying Sr2+ and Mg2+ sites with eight-coordination, and the third emission band with peak at 575 nm can be assigned to the 5d–4f transitions of Eu2+ occupying Y3+ sites with six-coordination, respectively.
000 cm−1); V is the valence of the Eu2+ ion (V = 2); n is the number of anions in the immediate shell around the Eu2+ ion; r is the effective radius of the host cation replaced by the Eu2+ ion (in Å); and “Ea” is the electron affinity of the anion atoms (in eV) dependent on the anion complex type. Here, Ea is approximately determined as 2.19 eV for the phosphate host.15 Table 3 show the experimental and calculated emission wavelengths of Eu2+ ions occupying different cation ions and coordination environments in the SMYP host. The result indicate that the experimental values agree well with the calculated values and the broad asymmetric yellow-emitting band is due to Eu2+ ions occupying eight-coordinated Sr2+ and Mg2+, and six-coordinated Y3+ site. Therefore, we can draw a conclusion that the first band centered at 432 nm (Eu1) is attributed to the 5d–4f transitions of Eu2+ occupying Sr2+ and Mg2+ sites with nine-coordination, and the second band centered at 504 nm is due to the 5d–4f transitions of Eu2+ occupying Sr2+ and Mg2+ sites with eight-coordination, and the third emission band with peak at 575 nm can be assigned to the 5d–4f transitions of Eu2+ occupying Y3+ sites with six-coordination, respectively.
| n | r (Å) | Ecalcd (cm−1) | λcalcd (nm) | λexp (nm) |
|---|---|---|---|---|
| 8 | rSr/1.26 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 264 264 |
470 | |
| 9 | rSr/1.31 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 566 566 |
443 | |
| 8 | rMg/0.89 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 652 652 |
536 | 432, 504, 575 |
| 8 | rZn/0.9 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 729 729 |
534 | 413, 504, 558 |
| 6 | rLa/0.9 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 894 894 |
592 |
Variations in the PLE and PL spectra of the Znm-SMYP: Eu2+ (0 ≤ m ≤ 1) samples depending on the m values are shown in Fig. 5. The results illustrate that all of the PLE spectra of the materials show characteristic excitation bands of Eu2+ ions, which are broadly in the range from 250 nm to 470 nm. Besides, the PLE spectra consist of three bands originating from the 4f–5d transition of Eu2+. The shoulders of the excitation spectra around 318 nm and 433 nm showed shifts toward shorter wavelength with increasing value of m. Upon the excitation of 375 nm, all of the samples exhibit the yellow emission bands, which are attributed to the 4f65d–4f7 transition of the Eu2+ ion. The emission peak position is almost constant, while the emission edge shifts toward a shorter wavelength as the Zn2+ concentration increases from m = 0 to m = 1.0. The experimental and calculated emission wavelengths of Eu2+ ions occupying different cation ions and coordination environments in the SZYP host are also shown in Table 3. At the same time, the emission intensities were gradually enhanced and maximized at the Zn2+ concentration of 0.8. Therefore, Zn0.8-SMYP: Eu2+ would be selected as the studied composition of the potential yellow-emitting phosphors for white LED applications in the present series.
With substitution of Mg2+ for Zn2+ (i.e., from SMYP: Eu2+ to SZYP: Eu2+), the emission edges of longer and shorter wavelength show obvious blue-shifting, whereas the emission peak do not show any obvious shifting. This observation could be explained in terms of two competing factors: the crystal field and the bond covalence.4,16
Continuous change of the emission edge wavelengths in PL spectra is observed with increasing Zn2+ concentration. This blue shift is partly ascribed to the changes in crystal field strength acting on the Eu2+ because the incorporation of Zn2+ ions into the lattice causes the distortion and changes the symmetry of host lattice, which results in a decrease of crystal field spitting for the 5d energy levels. According to reports by Robertson et al.17 and Jang et al.,18 crystal field splitting (Dq) can be determined by the following equation:19
 | (2) |
| m | Dq (cm−1) | PL intensity (%) | FWHM (nm) |
|---|---|---|---|
| 0 | 8352 | 68.9 | 150 |
| 0.2 | 8235 | 75.3 | 147 |
| 0.5 | 7961 | 76.8 | 145 |
| 0.8 | 7580 | 100 | 117 |
| 1 | 7538 | 90.7 | 104 |
In addition, the observed blue-shift could be explained in terms of the decreasing nephelauxetic effect.21 The nephelauxetic effect is a measure of the degree of covalency in the bonds between a metal ion and its surrounding ligands. Covalency is an important feature of the local structure.22 The observed emission from Eu2+ arises from the transition from the lower 4f65d state to the 4f7 (8S7/2) ground state. For the 4f65d configuration, the nephelauxetic effect markedly reduces the 4f–5d electrostatic interaction;23 covalency effects are primarily responsible for affecting the energy of the 4f65d1 excited level.24 Because the f-electrons are well shielded, the nephelauxetic effect on the 5d orbital of Eu2+ is expected to be very strong. As the Zn2+ concentration (m) increases, the interaction between the 5d electron of Eu2+ and the ligand decreases and shrinks the electron cloud of the 5d orbital of Eu2+.25 With the decrease in the degree of covalence of Eu–O bonds in the order of Mg and Zn, less negative charge transfer to Eu2+ ions and thus increase the energy difference between the 4f and the 5d levels.26 Thus, the degree of covalence in the Eu–O bonds was decreased with substitution of Mg2+ with smaller Zn2+ cations, and consequently can result in the blue shifting of the Eu2+ emission band. The strong nephelanxetic effect is also supported by the observed FWHMs for Znm-SMYP: Eu2+ phosphors, resulting in a decrease of the energy gravity between 4f and 5d energy levels.
As shown in Fig. 5, the emission peak position is almost constant within the range of 0 ≤ m ≤ 1. In contract, the shoulder position is shifted to shorter wavelength by increasing Zn occupancy. The chromaticity coordinates on commission international de I'Eclairage (CIE) 1931 of SMYP partial replaced by different concentrations of Zn2+ ions were calculated according to the emission curve as shown in Fig. 5. The change in the PL spectra in the range of m = 0–1.0 caused a marked change in emission color, as indicated by the shift in CIE color coordinates in Fig. 6. The chromaticity coordinates (x, y) shift from (0.355, 0.469) to (0.251, 0.451), when the Zn2+ concentration (m) varies from 0 to 1, corresponding to the hue converted from yellow to green, which was attributed to the emission intensity and peak position change under ultraviolet (UV) light of 375 nm. The results demonstrate that the Znm-SMYP: Eu2+ is a color tunable phosphor by changing the Zn/Mg ratio, which could be more flexible for the generation of white light by combination of other phosphors and LED chips.
 | ||
| Fig. 6 CIE chromaticity coordinates of Znm-SMYP: Eu2+ phosphors. (1) m = 0, (2) m = 0.2, (3) m = 0.5, (4) m = 0.8, and (5) m = 1.0. | ||
4. Conclusions
The ability to tune the color point of photoluminescence materials is particularly pertinent for practical applications. Here we have successfully synthesized the solid solution Sr8Mg1−mZnmY(PO4)7 (0 ≤ m ≤ 1) doped with 1 atom% Eu2+ and investigated the structural and luminescence properties. By partially substituting Mg2+ with Zn2+, a regular variation was found among the XRD patterns. With Zn2+ content increasing, the crystal field splitting between two lowest Eu2+ 5d levels decreased. As a result, the emission color alters from yellow to green. The decrease of the crystal field strength and the covalence results in a blue shift reflected in the luminescence spectra with the doping of Zn. The excitation spectra show broad band excitation in the 250–470 nm n-UV regions, which matches well with n-UV chips. The emission spectra exhibit strong tunable emissions color. All these results indicate that the Eu2+-doped Sr8(Mg,Zn)La(PO4)7 phosphor can be applied as a color-tunable phosphor for green or greenish white light emitting diode based on ultraviolet chip/phosphor technology.Acknowledgements
This research was financially supported by the National Pharmacy Experimental Teaching Centre of South-Central University for Nationalities (no. 406702).Notes and references
- H. A. Höppe, Angew. Chem., Int. Ed., 2009, 48, 3572 CrossRef PubMed; R. J. Xie, N. Hirosaki, T. Suehiro, F. F. Xu and M. Mitomo, Chem. Mater., 2006, 18, 5518 Search PubMed.
- M. Shang, C. Li and J. Lin, Chem. Soc. Rev., 2014, 43, 1372 RSC.
- M. Hannah, A. B. Piquette, M. Anc, J. Kittrick, J. Talbot, J. K. Han and K. Mishra, ECS Trans., 2012, 41, 19 RSC.
- P. Dorenbos, J. Lumin., 2003, 104, 239 CrossRef CAS.
- W. B. Im, N. George, J. Kurzman, S. Brinkley, A. Mikhailovsky, J. Hu, B. F. Chmelka, S. P. DenBaars and R. Seshadri, Adv. Mater., 2011, 23, 2300 CrossRef CAS PubMed.
- C. H. Huang, Y. C. Chen, T. M. Chen, T. S. Chan and H. S. Sheu, J. Mater. Chem., 2011, 21, 5645 RSC.
- C. H. Huang, Y. C. Chiu, Y. T. Yeh and T. M. Chen, Mater. Express, 2012, 2, 303 CrossRef CAS PubMed.
- C. H. Huang, D. Y. Wang, Y. C. Chiu, Y. T. Yeh and T. M. Chen, RSC Adv., 2012, 2, 9130 RSC.
- C.-H. Huang and T.-M. Chen, Inorg. Chem., 2011, 50, 5725 CrossRef CAS PubMed.
- T. Wanjun and C. Donghua, J. Am. Ceram. Soc., 2009, 92, 1059 CrossRef PubMed.
- T. Wanjun, Z. Fen and H. Shanshan, ECS J. Solid State Sci. Technol., 2014, 3(4), R65 CrossRef PubMed.
- A. R. Denton and N. W. Ashcroft, Phys. Rev. A: At., Mol., Opt. Phys., 1991, 43(6), 3161 CrossRef CAS; C. R. Volker Bachmann, O. Oeckler, W. Schnick and A. Meijerink, Chem. Mater., 2009, 21, 316 CrossRef.
- E. Nakazawa and F. Shiga, J. Lumin., 1977, 15, 255 CrossRef CAS.
- L. G. Van Uitert, J. Lumin., 1984, 29, 1 CrossRef CAS.
- C.-H. Huang, Y.-C. Chiu, Y.-T. Yeh, T.-S. Chan and T.-M. Chen, ACS Appl. Mater. Interfaces, 2012, 4, 6661 CAS.
- V. Bachmann, C. Ronda, O. Oeckler, W. Schnick and A. Meijerink, Chem. Mater., 2009, 21, 316 CrossRef CAS.
- J. M. Robertson, M. W. van Tol, W. H. Smits and J. P. H. Heyene, Philips J. Res., 1981, 36, 15 CAS.
- H. S. Jang, W. B. Im, D. C. Lee, D. Y. Jeon and S. S. Kim, J. Lumin., 2007, 126, 371 CrossRef CAS PubMed.
- P. D. Rack and P. H. Holloway, Mater. Sci. Eng., R, 1998, 21, 171 CrossRef.
- H. A. Hope, H. Lutz, P. Morys, W. Schnick and A. Seilmeier, J. Phys. Chem. Solids, 2001, 61, 2001 CrossRef CAS; R. J. Xie, N. Hirosaki, T. Suehiro, F. F. Xu and M. Mitomo, Chem. Mater., 2006, 18, 5578 CrossRef.
- N. K. Davidenk and K. B. Yatsimirskii, Theoretical and Experimental Chemistry, Springer, New York, 1973, p. 505 Search PubMed.
- R. Reisfeld, Struct. Bonding, 1973, 13, 53 CrossRef CAS.
- R. C. Alig, R. C. Duncan and B. J. Mokross, J. Chem. Phys., 1973, 59, 5837 CrossRef CAS PubMed.
- A. Diaz and D. A. Keszler, Chem. Mater., 1997, 9, 2071 CrossRef CAS.
- J. K. Park, C. H. Han, C. H. Kim, H. D. Park and S. Y. Choi, Electrochem. Solid-State Lett., 2001, 5, H11 CrossRef PubMed.
- T.-W. Kuo, W.-R. Liu and T.-M. Chen, Opt. Express, 2010, 18, 1888 CrossRef CAS PubMed; J. S. Kim, P. E. Jeon, J. C. Choi and H. L. Park, Solid State Commun., 2005, 133, 187 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2014 |