Study on the Atherton–Todd reaction mechanism†
V. Mitova,
N. Koseva and
K. Troev*
Institute of Polymers, Bulgarian Academy of Sciences, Acad. Georgi Bonchev Str., Bl. 103A, Sofia 1113, Bulgaria. E-mail: ktroev@polymer.bas.bg
First published on 14th November 2014
Abstract
A new mechanism of the Atherton–Todd reaction is discussed. The first step of the reaction between diesters of H-phosphonic acid and carbon tetrachloride in the presence of a base, commonly triethylamine, is a salt formation between carbon tetrachloride and the base [amine·Cl]+CCl3−. The trichloromethanide anion [CCl3−] deprotonates dialkyl H-phosphonate to form chloroform and dialkyl phosphonate anion [(RO)2P(O)]−. The latter anion reacts with the chlorine cation to furnish dialkyl chlorophosphate. Based on these findings the reaction has been applied for the oxidation of poly(alkylene H-phosphonate)s to the corresponding poly(alkylene chlorophosphate)s via the Atherton–Todd reaction.
1. Introduction
The Atherton–Todd reaction is one of the most characteristic reactions of diesters of H-phosphonic acid. It is a route for the oxidation of dialkyl H-phosphonates to the highly reactive dialkyl chlorophosphates.1,2The reaction is widely used in situ under mild conditions for the synthesis of a large number of biologically active compounds such as phosphates and amidophosphates.3 The Atherton–Todd reaction is a preferred method of synthesis of polyphosphoesters including poly[(hydroxy or alkyl)alkylene phosphate]s,4 poly(alkylene amidophosphate)s5 and polymer conjugates from poly(alkylene H-phosphonate)s.6–9 Recently, Atherton–Todd reaction has been used for direct azidation, cyanation and thiocyanation of diethyl or dii-buthyl H-phosphonates.10 The commonly proposed mechanism of the reaction is based primarily on the early kinetic investigations done by Steinberg.11 The initial step of the proposed two mechanisms involves deprotonation of dialkyl H-phosphonate (RO)2P(O)H by a base B to give the dialkyl phosphite anion, (RO)2PO−. This anion then reacts as a nucleophile with BCl+ to furnish the corresponding chlorophosphate (Scheme 1), or reacts with carbon tetrachloride (Scheme 2).
 | ||
| Scheme 1 Mechanism of Atherton–Todd reaction.11 | ||
 | ||
| Scheme 2 Mechanism of Atherton–Todd reaction.11 | ||
The validity of the deprotonation step in the above mechanism for the case of amine application is however questionable, since it has been established that amines are alkylated, but not protonated at the nitrogen by dialkyl H-phosphonates.12–14 Based on this observation it was accepted that the first step of Atherton–Todd reaction is alkylation of base (Scheme 3).15,16
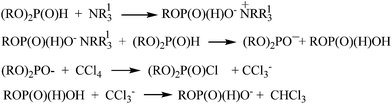 | ||
| Scheme 3 Mechanism of Atherton–Todd reaction.15,16 | ||
According to Krutikov et al.17 the mechanism of the synthesis of phosphoramidates via the Atherton–Todd reaction is based on the primary interaction of a polyhaloalkane with the highly basic amine to form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 associate. Authors accepted that the subsequent attack by the associate on the tricoordinated form of dalkyl H-phosphonate lead to the formation of the target compounds in high yields. In the review of Jaffres et al.18 devoted to the Atherton–Todd reaction are discussed all proposed mechanisms and the conclusion is that they led to controversial reports over the past years. In fact, till now there are no direct evidences for the proposed mechanisms.
1 associate. Authors accepted that the subsequent attack by the associate on the tricoordinated form of dalkyl H-phosphonate lead to the formation of the target compounds in high yields. In the review of Jaffres et al.18 devoted to the Atherton–Todd reaction are discussed all proposed mechanisms and the conclusion is that they led to controversial reports over the past years. In fact, till now there are no direct evidences for the proposed mechanisms.
In this paper we provide a direct evidence for Atherton–Todd reaction mechanism according to which the first step is a complex formation between base and carbon tetrachloride.
2. Results and discussion
We did experiments between dimethyl H-phosphonate and a secondary amine. It was established that the interaction of dimethyl H-phosphonate with dibutylamine at room temperature resulted in alkylation of dibutylamine.In the 31P{H}NMR spectrum of the reaction mixture there are signals for two types of phosphorus atoms at δ = 11.00 ppm and at 5.77 ppm. In the 31P NMR (see ESI Fig. 1†) spectrum the signal at 11.00 ppm represents a doublet of septets with 1J(P, H) = 697.65 Hz and 3J(P, H) = 11.86 Hz and is assigned to the phosphorus atom of unreacted dimethyl H-phosphonate. Those one at 5.77 ppm appears as a doublet of quartets with 1J(P, H) = 606.38 Hz and 3J(P, H) = 11.87 Hz. This signal can be assigned to the phosphorus atom of the mono-dealkylated H-phosphonate. The alkylation of dibutylamine is also confirmed and by 1H NMR spectrum. Besides the signals of the unreacted dimethyl H-phosphonate (doublets at 6.64 ppm with 1J(P, H) = 697.64 Hz and at 3.51 ppm with 3J(P, H) = 11.88 Hz for the protons in the P–H and P–OCH3 groups, respectively) there are new signals at δ = 6.64 ppm with 1J(P, H) = 606.35 Hz assigned to the P–H of mono-dealkylated H-phosphonate and at δ = 3.41 ppm with 3J(P, H) = 11.92 Hz attributed to the methoxy group of mono-dealkylated H-phosphonate. The singlet at 2.08 ppm can be assigned to N–CH3 protons.
When dimethyl H-phosphonate was added to in advanced mixed dibutylamine with carbon tetrachloride the NMR data showed that there proceeded the Atherton–Todd reaction (see ESI Fig. 2†). The reaction is highly exothermic and the corresponding amidophosphate is formed.
In the 31P{H}NMR spectrum (see ESI Fig. 2†) of the reaction mixture there are signals for two types of phosphorus atoms at δ = 13.73 ppm and at 11.02 ppm. In the 31P NMR spectrum the signal at 13.73 ppm represents 11 lines with 3J(P, H) = 11.18 Hz and can be assigned to the phosphorus atom of dimethyl dibutylamidophosphate. The one at 11.02 ppm appears as a doublet of septets with 1J(P, H) = 698.16 Hz and 3J(P, H) = 11.85 Hz and can be assigned to the phosphorus atom of dimethyl H-phosphonate. There is no signal in the range between 5 ppm and 6 ppm which is characteristic for the mono-dealkylated H-phosphonate. Expected dealkylation of dimethyl H-phosphonate did not occur.
The formation of complex between carbon tetrachloride and base (triethylamine) was confirmed by 1H NMR spectroscopy (See ESI Fig. 3†).
The signals for CH3 and CH2 protons in 1H NMR spectrum of the complex are shifted to low field compared to pure triethylamine. The triplet for CH3 protons and quartet for CH2 protons of pure triethylamine appears at 1.030 ppm and 2.524 ppm, those one of the complex appear at 1.035 ppm and 2.532 ppm, and in the salt at 1.042 ppm, and 2.543 ppm, respectively. Shifting for CH3 and CH2 protons of pure triethylamine compared to salt is 0.005 ppm (6.66 Hz) and 0.011 ppm (10.42 Hz), respectively.
Based on these results the following mechanism of Atherton–Todd reaction can be proposed (Scheme 4).
It can be assumed that the first step of the reaction is a salt formation between carbon tetrachloride and the base. Obviously, the formation of this salt is the main reason dibutylamine do not participate in a dealkylation reaction. Trichloromethanide anion [CCl3−] deprotonates dialkyl H-phosphonate to form chloroform and a dialkyl phosphonate anion [(RO)2P(O)]−. The latter anion takes the chlorine cation to furnish dialkyl chlorophosphate.
According to the proposed mechanism the Atherton–Todd reaction can be applied for oxidation of poly(alkylene H-phosphonate)s to the corresponding poly(alkylene chlorophosphate)s (Scheme 5).
 | ||
| Scheme 5 Reaction mechanism of the oxidation of poly(alkylene H-phosphonate)s via Atherton–Todd reaction. | ||
In the 31P{H}NMR spectrum of the mixture (See ESI Fig. 4a†) poly(oxyethylene H-phosphonate), triethylamine and CCl4 (Atherton–Todd reaction conditions) measured after 4 h, there are signals for the phosphorus atom in the repeating units at 9.85 ppm, a doublet of quintets (See ESI Fig. 4b†), and at 5.89 ppm, new one, a; quintet for the phosphorus atom connected with a chlorine atom (10% conversion). It can be accepted that the active species in the oxidation of poly(alkylene H-phosphonate)s via Atherton–Todd reaction to the corresponding poly(alkylene chlorophosphate)s is the phosphonate anion. The formation of the phosphonate anion can be formed via deprotonation of the poly(alkylene H-phosphonate) by the trichloromethanide anion.
3. Experimental
Materials
Dimethyl H-phosphonate, dibutylamine and triethylamine were purchased from Sigma-Aldrich and distilled prior to use. Carbon tetrachloride was obtained from Sigma-Aldrich, dried over P2O5 and distilled prior to use. All NMR spectra were measured on Bruker Avance II+ 600 NMR spectrometer in CDCl3.The poly(oxyethylene H-phosphonate) was obtained via polytransesterification of dimethyl H-phosphonate and poly(ethylene glycol) with number average molecular weight 600 g mol−1 (PEG 600) following a procedure described in ref. 4.
Interaction of dimethyl H-phosphonate with dibutylamine
1 ml of dimethyl H-phosphonate (0.0109 mol) and 1.84 ml dibutylamine (0.0109 mol) were mixed at room temperature under inert atmosphere (gentle flow of dry argon). After stirring the mixer for 48 h, sample was taken without further isolation for NMR analysis in CDCl3.31P{H}NMR (CDCl3), δ ppm: 11.00 ppm (content 39.5%); 5.77 ppm (content 60.5%).
31P NMR (CDCl3), δ ppm: 11.00 dseptets, 1J(P, H) = 697.65 Hz, 3J(P, H) = 11.86 Hz, CH3OP(O)(H)OCH3; 5.77 ppm, dq, 1J(P, H) = 606.38 Hz, 3J(P, H) = 11.87 Hz, CH3OP(O)(H)O−NHCH3+(C4H9)2.
1H NMR (CDCl3), δ ppm: 0.80 ppm, t, CH3–CH2–, 3J(H, H) = 7.2 Hz; 1.14–1.39 ppm, –CH2–CH2–CH3; 1.48–1.60 ppm, –CH2CH2–CH2–; 2.08 ppm, –N–CH3; 2.51–2.63 ppm, N–CH2–CH2–; 3.41 ppm, d, P–OCH3, 3J(P, H) = 11.92 Hz; 3.65 ppm, d, P–OCH3, 3J(P, H) = 11.88 Hz; 6.64 ppm, P–H, d, 1J(P, H) = 606.35 Hz, CH3OP(O)(H)O−NHCH3+(C4H9)2; 6.64 ppm, 1J(P, H) = 697.64 Hz, d, CH3OP(O)(H)OCH3.
Oxidation of dimethyl H-phosphonate via Athertton–Todd reaction
To a solution of 1.84 ml dibutylamine (0.0109 mol) in carbon tetrachloride (2.5 ml, 0.027 mol) at room temperature under inert atmosphere and stirring was added drop-wise 1 ml of dimethyl H-phosphonate (0.0109 mol). When dimethyl H-phosphonate was added to in advanced mixed dibutylamine with carbon tetrachloride, the reaction is highly exothermic. After stirring the mixer for 24 h, sample was taken without further isolation for NMR analysis in CDCl3 and dibutylamine were mixed (gentle flow of dry argon).31P{H}NMR (CDCl3), δ ppm: 13.73 ppm (content 59.5%); 11.02 ppm (content 41.5%).
31P NMR (CDCl3), δ ppm: 13.73 ppm, 11 lines, 3J(P, H) = 11.18 Hz, (CH3O)2P(O)N(C4H9)2; 11.02 ppm, dseptets, 1J(P, H) = 698.16 Hz, 3J(P, H) = 11.86 Hz, CH3OP(O)(H)OCH3.
1H NMR (CDCl3), δ ppm: 0.86 ppm, t, 3J(H, H) = 7.2 Hz, CH3–CH2–; 1.16–1.40 ppm, –CH2–CH2–CH3; 1.71–1.84 ppm, –CH2CH2–CH2–; 2.72–2.93 ppm, P–N–CH2–CH2–; 3.57 ppm, d, P–OCH3, 3J(P, H) = 11.1 Hz, (CH3O)2P(O)N(C4H9)2; 3.69 ppm, d, P–OCH3, 3J(P, H) = 11.88 Hz, (CH3O)2P(O) (H); 6.68 ppm, d, 1J(P, H) = 697.80 Hz, (CH3O)2P(O)(H).
Oxidation of poly(oxyethylene H-phosphonate) via Athetton–Todd reaction
The mixture of 0.035 g poly(oxyethylene H-phosphonate) based on PEG600 (0.054 mmol repeating units), 0.01 ml triethylamine (0.072 mmol) and 0.017 ml carbon tetrachloride (0.175 mmol) were mixed at room temperature under inert atmosphere (gentle flow of dry argon). After stirring the mixer for 24 h, sample was taken without further isolation for NMR analysis in CDCl3.31P{H}NMR (CDCl3), δ ppm: 9.87 ppm (content 88.4%); 5.89 ppm (content 11.6%).
31P NMR (CDCl3), δ ppm: 9.87 ppm, dquintets, 1J(P, H) = 715.84 ppm, 3J(P, H) = 9.11 Hz, –CH2O–P(O)(H)OCH2–; 5.89 ppm, quintet, 3J(P, H) = 9.11 Hz, –CH2O–P(O) (Cl)OCH2–;
Interaction of triethylamine with carbontetrachloride
60 mg of triethylamine (0.00059 mol) and 90 mg of carbon tetrachloride (0.00059 mol) were mixed at room temperature. After stirring for 48 h, the solid sample was taken without further isolation for NMR analysis in CDCl3.1H NMR (CDCl3), δ ppm: 1.042 ppm, t, CH3–, 3J(H, H) = 7.2 Hz; 2.53–2.56 ppm, N–CH2–CH3.
Acknowledgements
Financial support of this work was provided by the National Science Fund of Bulgaria (National Center for Advanced Materials (UNION) Module 2 “New materials in medicine and pharmacy” Contract DCVP 02-2/2009.References
- F. R. Atherton, H. T. Openshaw and A. R. Todd, J. Chem. Soc., 1945, 660–663 RSC.
- F. R. Atherton and A. R. Todd, J. Chem. Soc., 1947, 647–678 Search PubMed.
- K. D. Troev, Chemistry and Application of H-phosphonates, Elsevier, Amsterdam, NY, 2006, ch. 3, p. 39 Search PubMed.
- K. Troev, I. Tsacheva, N. Koseva, R. Georgieva and I. Gitsov, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 1349–1363 CrossRef CAS.
- K. D. Troev, Polyphosphoesters: Chemistry and Application, Elsevier, Amsterdam, NY, 2012, ch. 1, p. 49 Search PubMed.
- I. Pencheva, A. Bogomilova, N. Koseva, D. Obreshkova and K. Troev, J. Pharm. Biomed. Anal., 2008, 48, 1143–1150 CrossRef CAS PubMed.
- K. Troev, V. Mitova and I. Ivanov, Tetrahedron Lett., 2010, 51, 6123–6125 CrossRef CAS PubMed.
- A. Bogomilova, M. Hohn, M. Gunther, K. Troev, E. Wagner and L. Schreiner, Eur. J. Pharm. Sci., 2013, 50, 410–419 CrossRef CAS PubMed.
- V. Mitova, S. Slavcheva, P. Shestakova, D. Momekova, N. Stoyanov, G. Momekov, K. Troev and N. Koseva, Eur. J. Med. Chem., 2014, 72, 127–136 CrossRef CAS PubMed.
- E. Shi and C. Pei, Synthesis, 2004, 2995–2998 CrossRef CAS PubMed.
- G. J. Steinberg, J. Org. Chem., 1950, 637–647 CrossRef CAS.
- K. Troev and G. Borisov, Phosphorus Sulfur Relat. Elem., 1987, 29, 129–145 CrossRef CAS.
- K. Troev and D. M. Roundhill, Phosphorus Sulfur Relat. Elem., 1988, 32, 243–246 CrossRef.
- K. Troev, Rev. Heteroat. Chem., 1994, 11, 89–119 CAS.
- E. Georgiev, D. Max Roundhill and K. Troev, Inorg. Chem., 1992, 31, 1965–1968 CrossRef CAS.
- E. M. Georgiev, J. Kaneti, K. Troev and D. M. Roundhill, J. Am. Chem. Soc., 1993, 115, 10964–10973 CrossRef CAS.
- V. I. Krutikov, A. V. Erin and A. V. Krutikova, Zh. Obshch. Khim., 2012, 82, 713–718 Search PubMed.
- S. S. Le Corre, M. Berchel, H. C. Gourves, J. P. Haelters and P. A. Jaffres, Beilstein J. Org. Chem., 2014, 10, 1166–1196 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra10228b |
| This journal is © The Royal Society of Chemistry 2014 |





