Electrospun acid–base pair solid dispersions of quercetin
Jie Yan*a,
Yong-Hui Wub,
Deng-Guang Yu*c,
Gareth R. Williams*d,
Shang-Meng Huangb,
Wen Taoc and
Jun-Yi Sunc
aResearch Center for Analysis and Measurement, Donghua University, Shanghai 201620, China. E-mail: yanjie01@eyoul.com; Tel: +86-21-67792047
bThe Department of Mechanical Engineering, Guangxi Technological College of Machinery and Electricity, Nanning 530007, China
cSchool of Materials Science & Engineering, University of Shanghai for Science and Technology, Shanghai 200093, China. E-mail: ydg017@usst.edu.cn; Fax: +86-21-55270632; Tel: +86-21-55274069
dUCL School of Pharmacy, 29-39 Brunswick Square, London, WC1N 1AX, UK. E-mail: g.williams@ucl.ac.uk
First published on 28th October 2014
Abstract
In this work, electrospinning was used to fabricate core–shell nanofibers containing acid–base pairs. Quercetin was used as a model active ingredient. To exploit its solubility in basic conditions, a solution of quercetin, polyvinylpyrrolidone (PVP) and sodium hydroxide was used as the core fluid, and one consisting of citric acid and PVP as the shell fluid. Scanning and transmission electron microscopy demonstrated that core–shell nanofibers with linear morphologies were obtained, without beads or spindles. X-ray diffraction showed that quercetin was present in the fibers in an amorphous state; Fourier transform infrared spectroscopy indicated this may be a result of hydrogen bonding between the drug and the polymer matrix. In in vitro dissolution tests the drug was found to be released immediately when the fibers encountered an aqueous medium. There was no change in the pH of the medium after dissolution, as a result of the presence of the acid–base pair. This provides a new strategy for improving the dissolution behavior of poorly water-soluble drugs using polymeric nanostructures.
1. Introduction
Electrospinning is a facile technique which has been widely explored for the generation of polymer nanofibers and polymer-based nanocomposites over the past decade.1–5 The working fluid in a traditional single fluid electrospinning process is often a mixed solution of an active ingredient and a filament-forming polymer; suspensions of functional nanoparticles may also be used.6–10 The active ingredient must have sufficient solubility in the chosen solvent to give an effective therapeutic dose in the patient. The polymer should have good filament-forming properties and be soluble at an adequate concentration to permit the formation of fibers. This greatly limits the development of functional materials based on monolithic electrospun fibers, because only a limited number of polymer/solvent combinations yield fibers. Fortunately, coaxial electrospinning, in which two fluids can be co-electrospun simultaneously, can overcome this limitation because only one fluid need to be electrospinnable for the process to proceed successfully and generate nanofibers.11–13 Moreover, co-axial spinning provides fibers with “core–shell” structures and the possibility of spatially tailoring the locations of the components within the fibers.14–16Single fluid electrospinning processes have been widely reported for the development of novel solid dispersions (SDs) of poorly water soluble drugs, aiming to improve their dissolution properties.17–19 These nanofiber-based SDs include double-component SDs consisting of an active ingredient and a polymer matrix and multiple-component SDs containing a drug, a polymer, a surfactant, and a sweeter.17,20 The SDs produced could release the incorporated active ingredients immediately when they encountered water. However, it is impossible to develop this type of SD for some drugs owing to their very low solubility in the organic solvents that are suitable for preparing electrospinnable polymer solutions. Most recently, a coaxial electrospinning process has been demonstrated to provide a solution to this problem, through which a new type of SD in the form of core–shell nanofibers was prepared. In this process, an unspinnable core fluid consisting of acyclovir and polyvinylpyrrolidone (PVP) in a solution containing N,N-dimethylacetamide (DMAc) could be converted into fibers if the sheath fluid was spinnable.21
Poorly water-soluble drugs are used in over 40% of currently available medical products; 60% of active ingredients under development are also poorly water soluble.20,22 Many of these drugs are ionisable, and hence their solubility can be enhanced by varying the pH, or by salt formation. Examples include N-acetyl-p-aminophenol, acyclovir, tamoxifen, itraconazole and amiodarone.
Here we report a new type of electrospun acid–base pair SD (AB-SD) for poorly water-soluble active ingredients, which was fabricated using a coaxial electrospinning process. Quercetin was used as a model poorly soluble drug. It is a flavonoid found in many foods and plants and which has been explored for treating a range of different diseases such as high cholesterol, heart disease, diabetes, cancer, and so on.23 Quercetin is an acidic drug, and thus its solubility increases with pH. Polymer science has great potential to develop novel drug delivery systems, particularly those capable of controlled release.24,25 Hydrophilic polymers such as PVP, polyvinyl alcohol, polyethylene glycol and poly(ethylene oxide) have been widely explored as carriers of poorly water soluble drugs, with the aim of developing new types of SDs.26–28 In this work, PVP, one of the most widely used polymers in pharmaceutics, was selected as filament-forming matrix for nanofiber preparation.27,28
2. Experimental
2.1. Materials
Quercetin (purity >98%, no. MUST-12072505) was purchased from the Beijing Aoke Biological Technology Co. Ltd. (Beijing, China). PVP K60 (M = 360![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was purchased from the Shanghai Yunhong Pharmaceutical Aids and Technology Co., Ltd. (Shanghai, China). Citric acid monohydrate, sodium hydroxide, and anhydrous ethanol were purchased from the Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). All other chemicals used were analytical grade. Water was double distilled before use.
000) was purchased from the Shanghai Yunhong Pharmaceutical Aids and Technology Co., Ltd. (Shanghai, China). Citric acid monohydrate, sodium hydroxide, and anhydrous ethanol were purchased from the Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). All other chemicals used were analytical grade. Water was double distilled before use.
2.2. Coaxial electrospinning
The shell fluid consisted of 8.0 g of PVP K60 and 1.40 g citric acid monohydrate dissolved in 100 mL of ethanol. Two different core fluids were used in this study: one consisted of 8.0 g of PVP K60, 0.40 g sodium hydroxide, and 2.5 g quercetin in ethanol–water (50/50 v/v; 100 mL total volume), and the other of 8.0 g of PVP K60, 0.40 g sodium hydroxide, and 5.0 g quercetin in ethanol–water (50/50 v/v; 100 mL total volume).Two syringe pumps (KDS200 and KDS100, Cole-Parmer, Vernon Hills. IL, USA) and a high-voltage power supply (ZGF 60 kV/2 mA, Shanghai Sute Corp., Shanghai, China) were employed in electrospinning. A homemade concentric spinneret was used to conduct both single fluid (adjusting the core or shell fluid flow rate to zero mL h−1) and coaxial electrospinning processes. This spinneret was prepared by inserting a small stainless steel tube (27G, outer and inner diameters are 0.42 and 0.21 mm respectively) into a larger stainless steel tube (18G, outer and inner diameters of 1.25 and 0.84 mm). The inner tube protrudes 0.2 mm from the outer tube.
Experiments were recorded using a digital video recorder (PowerShot A490, Canon, Tokyo, Japan) under 11× magnification. After initial optimization, the applied voltage was fixed at 12 kV, and the nanofibers collected on aluminum foil at a distance of 15 cm. All other parameters are listed in Table 1. The products were dried for at least 24 h at 40 °C under vacuum (320 Pa) in a DZF-6050 electric vacuum drying oven (Shanghai Laboratory Instrument Work Co. Ltd, Shanghai, China) to facilitate the removal of residual organic solvent and moisture. They were then stored in a desiccator before characterization was undertaken.
| No. | Electrospinning process | Fluid flow rate (mL h−1) | Morphology | Size (μm) | |
|---|---|---|---|---|---|
| Shella | Core | ||||
| a The shell fluid consisted of 8.0 g of PVP K60 and 1.40 g citric acid monohydrate in 100 mL ethanol.b This core fluid consisted of 8.0 g of PVP K60, 0.40 g sodium hydroxide, and 2.5 g quercetin in 100 mL water–ethanol (50/50 v/v).c Core fluid consisted of 8.0 g of PVP K60, 0.40 g sodium hydroxide, and 5.0 g quercetin in 100 mL water–ethanol (50/50 v/v). | |||||
| F1 | Single fluid | — | 1.0b | — | — |
| F2 | Single fluid | 1.0 | — | Nanofibers | 0.63 ± 0.11 |
| F3 | Coaxial | 0.5 | 0.5b | Nanofibers | 0.74 ± 0.18 |
| F4 | Coaxial | 0.5 | 0.5c | Nanofibers | 0.76 ± 0.22 |
2.3. Characterization
Transmission electron microscope (TEM) images of the core–shell nanofibers F3 and F4 were recorded on a JEM 2100F field-emission instrument (JEOL, Tokyo, Japan). Samples for TEM were prepared by fixing a lacey carbon-coated copper grid directly onto the collector and electrospinning onto it.
The topographies of raw quercetin powder and fibers F3 and F4 were observed under cross-polarized light using an XP-700 polarized optical microscope (Shanghai Changfang Optical Instrument Co. Ltd, Shanghai, China).
In vitro dissolution tests were carried out according to the Chinese Pharmacopoeia, 2010 ed. Method II, a paddle method, was performed using a RCZ-8A dissolution apparatus (Tianjin University Radio Factory, Tianjin, China). Formulations containing equal amounts of quercetin (i.e. 30 mg raw powder, 244 mg of fiber F3 and 137 mg of F4) were placed in 900 mL of physiological saline (PS, 0.9 wt%) at 37 ± 1 °C. The instrument was set to stir at 50 rpm, providing sink conditions with C < 0.2Cs. At predetermined time points, 5.0 mL aliquots were withdrawn from the dissolution medium and replaced with fresh medium to maintain a constant volume. After filtration through a 0.22 μm membrane (Millipore, Billerica, MA, USA) and appropriate dilution with PS, the samples were analyzed at λmax = 371 nm using a UV/vis spectrophotometer (UV-2102PC, Unico Instrument Co. Ltd., Shanghai, China). The cumulative amount of quercetin released was calculated from the data obtained with a predetermined calibration curve. Experiments were carried out six times, and the cumulative percent released reported as mean values ± S.D.
3. Results and discussion
3.1. Strategy for the preparation of AB-SDs
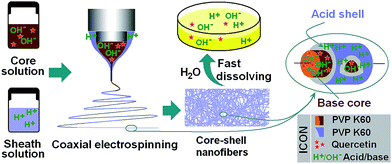
A diagrammatic sketch depicting the preparation of AB-SDs using coaxial electrospinning is shown in Fig. 1. The strategy comprises three steps: (1) to enhance the solubility of the drug, basic solutions of the drug and polymer were prepared, together with a separate solution containing an equal amount of citric acid and polymer; (2) coaxial electrospinning processes were carried out with the drug-free citric acid/polymer solution as the shell fluids to encapsulate the drug-containing core; and (3) the core–shell nanofiber AB-SDs were dried in an oven to remove solvent residues.Sharing characteristics of both electrospraying and conventional solution dryspinning, electrospinning can convert the working solutions into solid materials extremely rapidly, often on the time scale of 10−2 s.29,30 Electrospinning is thus an appropriate method for preparing nanocomposites.31 Coaxial electrospinning can duplicate the concentric structure of the spinneret into the nanoscale. The components in the shell and core fluids are propagated almost exclusively into the shell and core parts of the fibers. Electrospinning is also able to ‘freeze’ the drug molecules into a state comparable to a liquid form. This can be very useful to prevent phase separation or re-crystallization of drug.
3.2. The coaxial electrospinning process
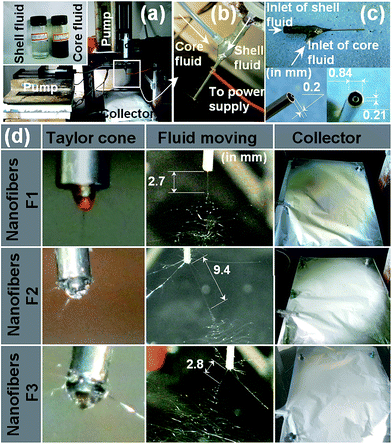
Photographs of the single fluid and coaxial electrospinning processes are given in Fig. 2. A digital image of the apparatus is illustrated in Fig. 2a; the inset shows the core and shell solutions. The connection between the power supply and the spinneret, and the connection between the spinneret with the core and shell syringe pumps are depicted in Fig. 2b and c.When the flow rate of the shell fluid was adjusted to 0 mL h−1, single fluid electrospinning of the core solution occurs. This process is depicted in Fig. 2d: the fluid behaviour is very typical, exhibiting a Taylor cone, followed by a straight jet and then a bending and whipping process. Single fluid electrospinning of the shell solution alone and co-axial electrospinning of the shell and core solutions also generated these typical fluid behaviours. However, there are several differences between them, which include: (1) the Taylor cone of the core fluid when spun alone was brown in colour, while that of the shell fluid alone was transparent. The compound Taylor cone in the coaxial process exhibited a yellowish-black colour, and the core solution is seen to be well encapsulated by the shell; (2) the straight fluid jets had different lengths: the core and shell fluids spun alone had lengths of 2.7 mm and 9.4 mm, respectively. Their combination in the coaxial process led to a jet of an intermediate length, 2.8 mm. (3) The fiber mats of F2 and F3 had a white colour, whereas those of F1 had a slight brown colour. This indicates that the quercetin-containing core was well encapsulated by the shell part in the core–shell F3 fibers.
After drying, the mats of F2, F3 and F4 could be easily peeled from the aluminium foil. However, F1 was strongly bound to the foil and could not be removed easily. This suggested that the solvent in the core fluid (most probably the water) did not fully evaporate during the electrospinning process.
3.3. Morphology and structure of the nanofibers
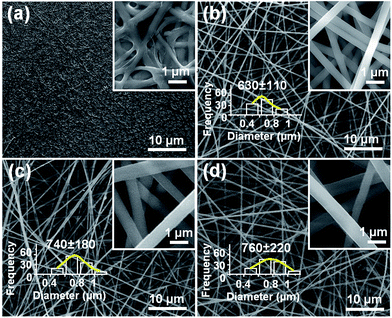
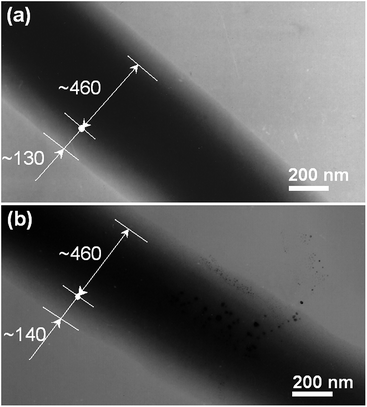
The morphologies of all four nanofiber formulations are shown in Fig. 3. Other than F1, the fibers have smooth surfaces and uniform structures without any beads-on-a-string or spindles-on-a-string morphologies. No drug nanoparticles could be discerned on the surface of F2, F3, or F4. The F2 fibers prepared by spinning the shell fluid alone had average diameters of 630 nm ± 110 nm (Table 1; Fig. 3b). The core–shell nanofibers F3 and F4 had average diameters of 740 nm ± 180 nm (Table 1; Fig. 3c) and 760 nm ± 220 nm (Table 1; Fig. 3d), respectively. Consistent with the observations detailed above in Section 3.2, F1 (from electrospinning of the core fluid alone) could not be totally solidified: residual solvent in the fiber mat led to their binding together on the collector, retaining vestiges of a fiber morphology only on the surface (Fig. 3a).Fig. 4 shows TEM images of the coaxial fibers F3 and F4. Both have clear core–shell structures, with the core and shell segments of each having similar sizes (the cores of both are estimated to be 460 nm and the shells 130 nm and 140 nm for F3 and F4, respectively). No particles could be discerned in the core of F3, suggesting a homogeneous structure (Fig. 4a). However, with the increased drug content of F4, solid phase separation was observed occasionally, as depicted in Fig. 4b. These of nanoparticles presumably formed during the electrospinning process and have penetrated both into the fiber shell and out the fibers.
3.4. Physical state and compatibility of components
The presence of numerous distinct reflections in its XRD pattern proves that the active ingredient quercetin exists as a crystalline material (see Fig. 5). This is also confirmed by the observation of colourful images when raw quercetin powders are viewed under polarized light. The quercetin crystals are chromatic under cross polarized light, while in sharp contrast the fibers shows no resolvable colour (Fig. 5).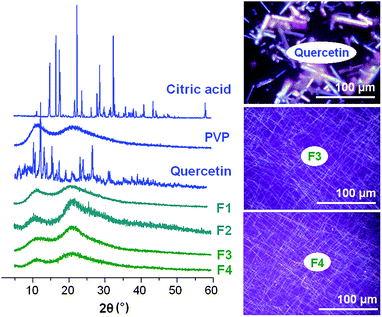 | ||
| Fig. 5 XRD patterns of the raw materials and fibers, and cross-polarized light observations of quercetin and F3 and F4. | ||
The XRD pattern of citric acid monohydrate also displays many Bragg reflections, demonstrating its existence as a crystalline material. The PVP diffraction pattern shows a diffuse background pattern with two diffraction halos, as expected given that it is an amorphous polymer.
The fibers F1 to F4 exhibit none of the characteristic reflections of the starting materials; instead their patterns comprise the diffuse haloes typical of amorphous materials. The combined XRD and light microscopy results clearly demonstrate that quercetin exists in an amorphous form in the fibers, losing its original crystalline nature. There is no evidence of crystalline material from the nanoparticles observed with F4; this might be because the amount is too small to be detected by XRD, or the nanoparticles may also be amorphous.
Compatibility among the components is very important for producing stable SDs with high quality. Often second-order interactions such as electrostatic interactions, hydrogen bonding, and hydrophobic interactions are desired for improving compatibility. The molecular structures of the main components used in this work are given in Fig. 6. Quercetin and citric acid molecules possess free hydroxyl groups which could act as potential proton donors for hydrogen bonding. PVP can act as a proton acceptor due to the presence of numerous carbonyl groups in its molecules, which result in a characteristic peak at 1671 cm−1 in its FTIR spectrum (Fig. 6). Therefore it can be postulated that hydrogen bonding should occur within the nanofibers, both in the inner core and in the outer shell.
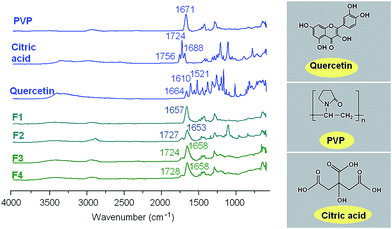 | ||
| Fig. 6 ATR-FTIR spectra of the raw materials and fibers, and the molecular structures of PVP, quercetin and citric acid. | ||
Quercetin has a characteristic peak of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups at 1664 cm−1 and the three peaks of benzene rings : two main peaks at 1610, 1521 cm−1, and a small peak between these. However, all these peaks were absent in the spectra of the nanofibers F1, F3 and F4. Only a single broad peak at 1653, 1658 and 1658 cm−1 can be identified for them, respectively, which shows a slight red shift compared to the spectrum of pure PVP (Fig. 6). Additionally, almost all peaks in the fingerprint regions of quercetin have shifted, decreased in intensity, or totally disappeared in the nanofibers' spectra. The above-mentioned phenomena suggest that hydrogen bonding occurs between the PVP carbonyl group and the hydroxyl group of the quercetin molecules in nanofibers F1 and the core parts of nanofibers F3 and F4.
O groups at 1664 cm−1 and the three peaks of benzene rings : two main peaks at 1610, 1521 cm−1, and a small peak between these. However, all these peaks were absent in the spectra of the nanofibers F1, F3 and F4. Only a single broad peak at 1653, 1658 and 1658 cm−1 can be identified for them, respectively, which shows a slight red shift compared to the spectrum of pure PVP (Fig. 6). Additionally, almost all peaks in the fingerprint regions of quercetin have shifted, decreased in intensity, or totally disappeared in the nanofibers' spectra. The above-mentioned phenomena suggest that hydrogen bonding occurs between the PVP carbonyl group and the hydroxyl group of the quercetin molecules in nanofibers F1 and the core parts of nanofibers F3 and F4.
Citric acid has three characteristic peaks of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups at 1756, 1724 and 1688 cm−1 resulting from different stretching vibrations of carbonyl groups in the raw crystalline material (Fig. 6). However, only two characteristic peaks appeared in the spectra of nanofibers F2, F3 and F4. One peak at 1727, 1724 and 1728 cm−1 cm−1 in the spectra of nanofibers F2, F3 and F4, respectively, is attributed to the stretching vibrations of free carbonyl groups in the loaded citric acid molecules. The other peak at 1653, 1658 and 1658 cm−1 suggests that hydrogen bonding occurs between the PVP carbonyl group and the hydroxyl group of the citric acid molecules in nanofibers F2 and the shell parts of nanofibers F3 and F4. The peak at 1658 cm−1 of nanofibers F3 and F4 is thought to be superposition of their core and shell FTIR responses.
O groups at 1756, 1724 and 1688 cm−1 resulting from different stretching vibrations of carbonyl groups in the raw crystalline material (Fig. 6). However, only two characteristic peaks appeared in the spectra of nanofibers F2, F3 and F4. One peak at 1727, 1724 and 1728 cm−1 cm−1 in the spectra of nanofibers F2, F3 and F4, respectively, is attributed to the stretching vibrations of free carbonyl groups in the loaded citric acid molecules. The other peak at 1653, 1658 and 1658 cm−1 suggests that hydrogen bonding occurs between the PVP carbonyl group and the hydroxyl group of the citric acid molecules in nanofibers F2 and the shell parts of nanofibers F3 and F4. The peak at 1658 cm−1 of nanofibers F3 and F4 is thought to be superposition of their core and shell FTIR responses.
PVP compounds are excellent auxiliaries for the manufacture of effective solid solutions and dispersions by traditional methods; it has been reported they can enhance the dissolution rates of over 140 poorly water-soluble drugs, and they often exhibit strong ability to inhibit crystallization of dispersed drugs.32,33 Here, quercetin molecules, by interacting with the polymer PVP, are less likely to form the dimers which are essential for formation of a crystal lattice. This should in turn enhance the stability of these structural nanoproducts for preservations.
3.5. In vitro dissolution tests
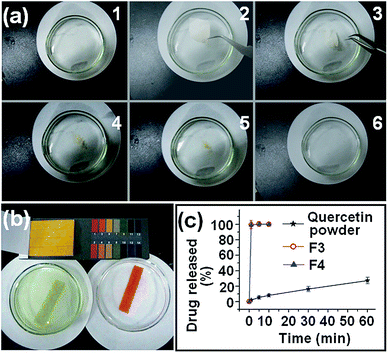
The fibers of F3 and F4 disappeared instantly after they were placed in the dissolution medium. This effect is attributed to the PVP polymer matrix being highly hygroscopic and hydrophilic, while in addition the nanofibers have very large surface areas, small diameters, and exist as a porous web structure. Finally, the encapsulated quercetin exists in the amorphous form, meaning there is no lattice energy barrier to dissolution. Photographs of the dissolution process of F4 are given in Fig. 7a. The entire process was complete in 18.7 ± 3.4 s (n = 3). When the F4 fibers were totally dissolved, the dissolution medium was neutral with a pH value of 6.9 (see Fig. 7b, left side). In contrast, the dissolution solution from F2 was acidic with a pH value of 3.2 (Fig. 7b, right side).Since quercetin has a UV absorbance peak at λmax = 371 nm, the amount of quercetin released from the fibers is easily determined by UV spectroscopy using a predetermined calibration curve: C = 15.95A − 0.0017 (R2 = 0.9997), where C is the quercetin concentration (μg mL−1) and A is the solution absorbance at 371 nm (linear range: 2 μg mL−1 to 20 μg mL−1).
The in vitro drug release profiles of F3, F4 and the raw quercetin powder (particle size smaller than 100 μm) are provided in Fig. 7c. The core–shell nanofibers F3 and F4 exhibited extremely rapid release of the incorporated quercetin, freeing all the drug within one minute. In comparison, the crude quercetin particles dissolved slowly, only reaching 28.9% release in one hour. Although the nanofibers F3 and F4 had different weights and different diameters, they were able to release all the incorporated drugs at similar speeds, within one minute. This suggests that the amorphous state of quercetin in the core–shell SDs played a dominant role during the in vitro dissolution processes, letting the drug synchronously dissolve with the polymer matrix through an erosion mechanism. Additionally, the final release amounts of quercetin from the core–shell nanofibers F3 and F4 were 30.2 ± 1.3 mg and 30.1 ± 1.1 mg, respectively, almost equivalent to the calculated value, indicating no drug loss during the coaxial electrospinning processes.
Accelerated dissolution of drugs, particularly those with poor water solubility, is highly sought after to enhance their pharmaceutical applications.20,21,34 The high surface area, narrow diameters and high porosity of electrospun fiber mats have been explored for different types of applications where rapid onset of action is required.10,35 This work opens a route for developing fast dissolving drug delivery systems. The AB-SDs of quercetin produced here could be further processed into fast disintegrating membranes for sublingual drug delivery or capsules for oral administration. It should be noted that, because quercetin is fragile under basic conditions,36 its chemical and physiological stability, and the physical stability of AB-SDs, should be systematically investigated before the development of commercial products.
4. Conclusion
The dissolution of poorly water-soluble drugs is one of the most challenging aspects in the development of new medicines. In this work, a new type of acid–base pair solid dispersion (AB-SDs) was developed to enhance the dissolution rate of an acidic drug. Poly(vinylpyrrolidone) was used as the polymer matrix and quercetin as a model drug, and AB-SDs of quercetin in the form of core–shell nanofibers were fabricated using coaxial electrospinning. Scanning electron microscopy images showed that the nanofibers were linear without any beads-on-a-string morphology. Transmission electron microscopy images revealed the fibers to have clear core–shell structures. X-ray diffraction demonstrated that the active ingredient was present in the fibers in an amorphous state, which might be attributed to the rapid evaporation of solvent in the electrospinning process and also the favourable secondary interactions between the drug and the polymer matrix suggested by infrared spectroscopy. In in vitro dissolution tests, the drug was released immediately when the core–shell nanofibers encountered the dissolution medium. When the AB-SD fibers were added to deionised water, the medium remained neutral after dissolution due to the presence of an acid–base pair in the fibers. This study therefore demonstrates the systematic design, preparation, characterization and application of a new type of SD to improve the dissolution properties of poorly water soluble drugs.Acknowledgements
This work was supported by the National Science Foundation of China (no. 51373101), the China NSFC/UK Royal Society Cost Share International Exchanges Scheme (nos 51411130128/IE131748), the Natural Science Foundation of Shanghai (no. 13ZR1428900) and the Key Project of the Shanghai Municipal Education Commission (no. 13ZZ113).Notes and references
- G. S. Anjusree, A. Bhupathi, A. Balakrishnan, S. Vadukumpully, K. R. V. Subramanian, N. Sivakumar, S. Ramakrishna, S. V. Nair and A. S. Nair, RSC Adv., 2013, 3, 16720–16727 RSC.
- E. Atabey, S. Wei, X. Zhang, H. Gu, X. Yan, Y. Huang, L. Shao, Q. He, J. Zhu, L. Sun, A. S. Kucknoor, A. Wang and Z. Guo, J. Compos. Mater., 2013, 47, 3175–3185 CrossRef CAS PubMed.
- S. L. Liu, Y. Z. Long, Y. Y. Huang, H. D. Zhang, H. W. He, B. Sun, Y. Q. Sui and L. H. Xia, Polym. Chem., 2013, 4, 5696–5700 RSC.
- J. Sharma, X. Zhang, T. Sarker, X. Yan, L. Washburn, H. Qu, Z. Guo, A. Kuckoor and S. Wei, Polymer, 2014, 55, 3261–3269 CrossRef CAS PubMed.
- J. Zhu, M. Chen, Q. He, L. Shao, S. Wei and Z. Guo, RSC Adv., 2013, 3, 22790–22824 RSC.
- F. Zheng, S. Wang, M. Shen, M. Zhu and X. Shi, Polym. Chem., 2013, 4, 933–941 RSC.
- C. Su, Y. Tong, M. Zhang, Y. Zhang and C. Shao, RSC Adv., 2013, 3, 7503–7512 RSC.
- C. L. Zhang and S. H. Yu, Chem. Soc. Rev., 2014, 43, 4423–4448 RSC.
- H. Yang, P. F. Gao, W. B. Wu, X. X. Yang, Q. L. Zeng, C. Li and C. Z. Huang, Polym. Chem., 2014, 5, 1965–1975 RSC.
- D. G. Yu, F. Liu, L. Cui, Z. P. Liu, X. Wang and S. W. A. Bligh, RSC Adv., 2013, 3, 17775–17783 RSC.
- A. K. Moghe and B. S. Gupta, Polym. Rev., 2008, 48, 353–377 CrossRef CAS.
- P. Zhang, C. Shao, X. Li, M. Zhang, X. Zhang, C. Su, N. Lu, K. Wang and Y. Liu, Phys. Chem. Chem. Phys., 2013, 15, 10453–10458 RSC.
- S. Agarwal, A. Greiner and J. H. Wendorff, Prog. Polym. Sci., 2013, 38, 963–991 CrossRef CAS PubMed.
- C. Li, Z. H. Wang, D. G. Yu and G. R. Williams, Nanoscale Res. Lett., 2014, 9, 258 CrossRef PubMed.
- D. G. Yu, J. H. Yu, L. Chen, G. R. Williams and X. Wang, Carbohydr. Polym., 2012, 90, 1016–1023 CrossRef CAS PubMed.
- H. Chen, N. Wang, J. Di, Y. Zhao, Y. Song and L. Jiang, Langmuir, 2010, 26, 11291–11296 CrossRef CAS PubMed.
- Z. K. Nagy, A. Balogh, G. Drávavölgyi, J. Ferguson, H. Pataki, B. Vajna and G. Marosi, J. Pharm. Sci., 2013, 102, 508–517 CrossRef CAS PubMed.
- W. Lu, J. Sun and X. Jiang, J. Mater. Chem. B, 2014, 2, 2369–2380 RSC.
- D. G. Yu, X. X. Shen, C. Brandford-White, K. White, L. M. Zhu and S. W. A. Bligh, Nanotechnology, 2009, 20, 055104 CrossRef PubMed.
- D. G. Yu, J. M. Yang, C. Branford-White, P. Lu, L. Zhang and L. M. Zhu, Int. J. Pharm., 2010, 400, 158–164 CrossRef CAS PubMed.
- D. G. Yu, L. M. Zhu, C. Branford-White, J. H. Yang, X. Wang, Y. Li and W. Qian, Int. J. Nanomed., 2011, 6, 3271–3280 CrossRef CAS PubMed.
- H. Chen, J. Wan, Y. Wang, D. Mou, H. Liu, H. Xu and X. Yang, Nanotechnology, 2008, 19, 375104 CrossRef PubMed.
- H. L. Li, X. B. Zhao, Y. K. Ma, G. X. Zhai, L. B. Li and H. X. Lou, J. Controlled Release, 2009, 133, 238–244 CrossRef CAS PubMed.
- S. Demirci, A. Celebioglu, Z. Aytac and T. Uyar, Polym. Chem., 2014, 5, 2050–2056 RSC.
- L. Cui, Z. P. Liu, D. G. Yu, S. P. Zhang, S. W. A. Bligh and N. Zhao, Colloid Polym. Sci., 2014, 292, 2089–2096 CAS.
- X. Hu, S. Liu, G. Zhou, Y. Huang, Z. Xie and X. Jing, J. Controlled Release, 2014, 185, 12–21 CrossRef CAS PubMed.
- T. Vigh, T. Horváthová, A. Balogh, P. L. Sóti, G. Drávavögyi, Z. K. Nagy and G. Marosi, Eur. J. Pharm. Sci., 2013, 49, 595–602 CrossRef CAS PubMed.
- M. Chen, H. Qu, J. Zhu, Z. Luo, A. Khasanov, A. S. Kucknoor, N. Haldolaarachchige, D. P. Young, S. Wei and Z. Guo, Polymer, 2012, 53, 4501–4511 CrossRef CAS PubMed.
- O. V. Salata, Curr. Nanosci., 2005, 1, 25–33 CrossRef CAS.
- D. Li and Y. Xia, Adv. Mater., 2004, 16, 1151–1170 CrossRef CAS.
- X. Lu, W. Zhang, C. Wang, T. C. Wen and Y. Wei, Prog. Polym. Sci., 2011, 36, 671–712 CrossRef CAS PubMed.
- V. Bühler, Kollidon: Polyvinylpyrollidone for the Pharmaceutical Industry, BASF Aktiengesellschaft Feinchemie, Ludwigshafen, 2nd edn, 1998 Search PubMed.
- C. Leuner and J. Dressman, Eur. J. Pharm. Biopharm., 2000, 50, 47–60 CrossRef CAS.
- J. L. Manasco, C. Tang, N. A. Burns, C. D. Saquing and S. A. Khan, RSC adv., 2014, 4, 13274–13279 RSC.
- G. Fu, Z. Su, X. Jiang and J. Yin, Polym. Chem., 2014, 5, 2027–2034 RSC.
- Y. J. Moon, L. Wang, R. DiCenzo and M. E. Morris, Biopharm. Drug Dispos., 2008, 29, 205–217 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |