Determination of trace analytes based on diffuse reflectance spectroscopic techniques: development of a multichannel membrane filtration-enrichment device to improve repeatability
Wei Lia,
Lei Wanga,
Peijin Tonga,
Jibran Iqbalb,
Xiaofang Zhanga,
Xuan Wanga and
Yiping Du*a
aShanghai Key Laboratory of Functional Materials Chemistry, Research Centre of Analysis and Test, East China University of Science and Technology, Shanghai 200237, P. R. China. E-mail: yipingdu@ecust.edu.cn; Fax: +86-21-64252947T; Tel: +86-21-64250551
bInterdisciplinary Research Centre in Biomedical Materials, COMSATS Institute of Information Technology, Lahore 54000, Pakistan
First published on 8th October 2014
Abstract
The determination of trace analytes based on membrane filtration-enrichment and diffuse reflectance spectroscopic techniques has gained increasing interest in the past decade due to its simplicity, rapidity and high sensitivity. However, poor repeatability primarily attributed to the differences of characteristics between membrane filters limits the development of this technique. In the current study, a simple and effective multichannel device is specially designed for the membrane filtration-enrichment process. The device is able to enrich six samples simultaneously on different positions of a membrane filter and allows the spectroscopic measurement of six samples with only one membrane filter. The proposed approach avoided the effects caused by the nonuniform membrane filters on the performance of the enrichment process. Accuracy and repeatability have been improved significantly for the subsequent on-line spectroscopic detection. A case study was carried out to assess this method utilizing the carcinogenic dye rhodamine B (RhB) as a model analyte. Under the optimal conditions, linearity of the calibration curve based on the Kubelka–Munk function was achieved in the concentration range of 2–30 μg L−1 with the correlation coefficient (R2) of 0.9924. Good repeatability was achieved with three average relative standard deviation (RSD) values of 3.6%, 3.8% and 3.8% corresponding to the solutions of 30, 10 and 5 μg L−1 RhB, respectively. The presented method was successfully employed to quantify RhB in soft drink and river water samples.
1. Introduction
With the development of science and technology, analytical instruments have been very much improved both in sensitivity and accuracy. However, pretreatment methods still present some issues in the determination of trace analytes, due to the low concentrations of analytes in samples together with the high concentrations of interfering matrix components. There are a variety of methods for the separation and pre-concentration of analytes from sample matrices, such as liquid–liquid extraction (LLE),1–3 liquid–liquid microextraction (LLME),4–6 solid-phase extraction (SPE)7–9 and solid-phase microextraction (SPME).10–12Membrane filtration (Microfiltration) is a pressure driven SPE technique, which is usually applied to separate and enrich micron-sized and low concentration particles from fluids with microscopic pores (0.1–10 micrometer) membrane filters. It has received considerable attention and has been widely used for the pre-concentration of trace metallic ions after chromogenic reactions, owing to its simplicity of operation, rapidity, low cost, and high enrichment efficiency.13–20 The membrane filtration-enrichment technique also has a great potential to directly enrich organic compounds.21–23 Among the researches published over the last decade, the common way for the subsequent detection after the membrane filtration-enrichment procedure was dissolving the membrane filter concentrated analytes or eluting the analytes concentrated on the membrane filter with suitable reagents, and loading the reconstituted sample to atomic spectrometer (AAS or ICP)13,18,19,24 or measuring its ultraviolet-visible (UV-vis) spectrum.22,23,25 Some researchers,5,16,26,27 including our group,14,15,21 simplified the detection process by omitting the elution step and directly measuring the diffuse reflectance spectrum of the membrane filter concentrated analytes, i.e. the on-line spectroscopic determination. It avoided the use of eluents and the influence caused by elution operation. Unfortunately, poor reproducibility was introduced by the differences between membrane filters.5,26
The mechanism of the membrane filtration-enrichment is primarily sieving when the size of particles of the concentrated compound is larger than the pore size of the membrane filter used. If a particle is not much smaller than the pore, then as it passes near the pore there is a definite probability that part of the particle will touch the filter matrix. If adhesive forces are strong enough, the particle will be trapped. In the case of smaller particles, it is presumably attributed to adsorption to the filter surface and in the pore, which is caused by the interactions, including van der Waals forces, electrostatic interaction, hydrophobic interaction, π–π interaction and hydrogen bonding, between the analyte molecule and the filter matrix.28–30 Therefore, the characteristics of the membrane filter, such as major ingredient, pore density, pore size distribution, wettability, thickness and isotropy play decisive roles in the membrane filtration-enrichment process. Small differences of characteristics between membrane filters affect not only the efficiency and repeatability of enrichment process, but also the accuracy and repeatability of the subsequent detection, especially when on-line measuring the diffuse reflectance spectrum of the membrane filter for quantitative analysis. The way adopted to solve this problem in our previous work was meticulously selecting the membrane filters before all experiments by comparing their UV-vis spectra to obtain as uniform membrane filters as possible.
In the present work, a very simple but effective homemade multichannel device was designed and made for the membrane filtration-enrichment process, which contains six channels and can enrich six samples simultaneously on different positions of a membrane filter. The main purpose of using this device is to avoid the effects caused by the differences of characteristics between membrane filters which ultimately enhance the performance of the membrane filtration-enrichment process as well as the accuracy and repeatability of the subsequent on-line detection. In addition, batch membrane filtration-enrichment operation can easily be implemented by designing a solution importer with suitable number of channels. Rhodamine B (RhB) that is banned as a food additive by current legislation in many countries because of its carcinogenicity, was used as a model analyte to evaluate the effectiveness of the presented method.
2. Experimental
2.1 Chemicals and reagents
All chemical and reagents used were of analytical purity grade and used without further purification. Rhodamine B was purchased from Shanghai Yuanye biology and Science Co., Ltd. Mixed cellulose ester membrane filter, cellulose acetate membrane filter, nylon membrane filter and polytetrafluoroethylene (hydrophilic) membrane filter used in the present study were purchased from Shanghai Xingya purification materials factory with the pore size of 0.1 μm, 50 mm in diameter.A standard stock solution of RhB with the concentration of 1000 μg mL−1 was prepared by dissolving the powder RhB with ultra-pure water and stored in a refrigerator. All sample solutions were obtained by stepwise diluting the stock solution with ultra-pure water.
2.2 Apparatus
A miniature fiber optic UV-visible spectrometer USB2000+ (Ocean Optics (Shanghai) Co., Ltd) equipped with an integrating sphere accessory was used to collect the UV-vis spectral data from the membrane filter. A model PHS-25 pH meter (Mettler Toledo instrument (Shanghai) Co., Ltd) was used for pH measurements. Ultra-pure water was obtained from an ultra-pure water purification system (SARTORIUS arium 611DI, Germany, 18.2 MΩ* cm). Liquid chromatography mass spectrometer (LC-MS, Waters, Xevo G2-S QTof, USA) was utilized to detect the content of RhB for evaluating the enrichment efficiency. SHB-III Vadose water vacuum pump (Shanghai Weikai instrument equipment Co., Ltd, China) was used for the membrane filtration-enrichment.The membrane filtration-enrichment process was carried out by a homemade multichannel membrane filtration-enrichment device comprising an acrylic multichannel solution importer (Fig. 1(b)) and a commercial filtration device with a filter element of 42 mm in diameter (Tianjin linghang Technologies Co., Ltd, China) (Fig. 1(a)). As shown in Fig. 1, the membrane filter is placed between the multichannel solution importer and the filtration device. The multichannel solution importer is composed of a disk containing six holes and six acrylic pipelines, the disk is about 58 mm in diameter and 5 mm in height. The holes are 7 mm in diameter and evenly distribute within the disk. The pipelines are 50 mm in height and fit with the holes, respectively. The proposed device can enrich six samples on different positions of a membrane at the same time or in a certain order. The number of channels could be adjusted to smaller or larger than six accordingly.
2.3 Experiment procedure
For the membrane filtration-enrichment procedure under the optimal conditions: an aliquot of 20 mL sample solution containing the analyte RhB (pH = 1) was loaded into the six-channel membrane filtration-enrichment device and passed through the mixed cellulose esters membrane filter under suction with a vacuum pump at vacuum degree of 0.04 MPa. The analyte RhB was adsorbed by the membrane filter because of the interactions, including van der Waals forces, electrostatic interaction, and hydrogen bonding, between the RhB molecule and the filter matrix. After completion of filtration, the membrane filter was air-dried and the UV-vis diffuse reflectance spectrum was on-line measured for quantitative analysis. The analysis time of one sample including enrichment and spectral measurement was within 6 min.2.4 Quantitative basics of the membrane filtration-enrichment method
In diffuse reflectance spectroscopy analysis, the diffuse reflectance R∞ can be expressed as eqn (1):31
 | (1) |
| K = εC | (2) |
It is obvious that R∞ is nonlinear with C. The diffuse reflection absorbance A is frequently used to obtain a linear model and it is defined as eqn (3):
| A = log(1/R∞) | (3) |
Assuming S as a constant, and when C is limited in a narrow range, eqn (3) can be written as eqn (4).
| A = a + bK/S = a + bεC/S = a + dC | (4) |
The Kubelka–Munk function is another beneficial equation to link R∞ and C, which will also yield a linear model, and it reads as eqn (5):32
| F(R∞) = (1 − R∞)2/2R∞ = K/S = εC/S = bC | (5) |
The following equation can be derived from eqn (3) and (5):
| F(R∞) = (1 − 10−A)2/2 × 10−A = K/S = εC/S = bC | (6) |
In this study, the concentration of the analyte C in the above eqn indicates the concentration of RhB absorbed on the membrane filter. As the enrichment efficiency of the proposed method was nearly 100 percent (see section 3.6), the concentration of RhB absorbed on the membrane filter C is proportional to its concentration in the solution c. Thus C in the above eqn can be represented by c (Here it is supposed that the mass of effective membrane filter utilized to concentrate one sample is the same among all filtrations).
3. Result and discussion
3.1 Comparison of repeatability
The goal of developing the multichannel device that fulfills the enrichment of six samples on one membrane filter was to improve the repeatability of the membrane filtration-enrichment method. To compare the repeatability between the conventional method and this method, a series of experiments were conducted with 30 μg L−1 RhB solutions. For a more reasonable comparison, the ratio of the sample volume was stipulated to equal the ratio of the effective area of the filter element, because the multichannel device provided a smaller effective area for sample solution passing through than that of the conventional method. The effective diameter of the filter element of the conventional filtration system and the multichannel device were 42 mm and 7 mm, therefore the volumes of sample solutions were 288 mL and 8 mL, respectively. Five sample solutions of 288 mL were filtered with five randomly selected membrane filters using the conventional filtration system at vacuum degree of 0.098 MPa. Then UV-vis diffuse reflectance spectra of each membrane filter were measured from different positions. The mean values and the relative standard deviations (RSDs) of absorbance at 554 nm are listed in Table 1. Meanwhile, another five membrane filters were selected randomly, for each membrane filter, six sample solutions of 8 mL were filtered through six holes, respectively, using the developed six-channel device under same experimental conditions. The mean values and the RSDs of absorbance at 554 nm of the six samples on each membrane filter are also listed in Table 1.| No.a | Conventional method | This method | ||
|---|---|---|---|---|
| Absorbanceb | RSD% | Absorbancec | RSD% | |
| a Experiments with different membrane filters.b Average of ten UV-vis diffuse reflectance spectra of different positions on each membrane filter (same sample).c Average of six different samples on each membrane filter. | ||||
| 1 | 0.1551 | 4.7 | 0.1140 | 4.2 |
| 2 | 0.2037 | 4.1 | 0.1242 | 4.2 |
| 3 | 0.1528 | 1.6 | 0.1385 | 4.5 |
| 4 | 0.1969 | 3.2 | 0.1920 | 4.9 |
| 5 | 0.1430 | 2.2 | 0.1442 | 2.3 |
| Mean | 0.1703 | 0.1426 | ||
| RSD% | 16.4 | 21.1 | ||
Table 1 shows the different absorbances and RSDs for different membrane filters, which indicate the differences between membrane filters, while values of RSD on a row evaluate different positions on one membrane filter. The RSDs of the mean absorbance of each filter also reflect the differences between membrane filters.
For the conventional method, the RSDs of the absorbance between positions on each membrane filter are within the range of 1.6–4.7%. However, the RSD between membrane filters is high to 16.4%. It demonstrates that the differences between membrane filters have a strong impact on the repeatability of the membrane filtration-enrichment method.
When conducted experiments on one membrane filter with the proposed multichannel device, the RSDs of six different samples on each membrane filter were less than 4.9%, which is equivalent to the results using the conventional method (1.6–4.7%), although the RSD between membrane filters is still high (21.1%). It is obvious that there is a significantly improvement in the repeatability compared with the conventional method. It indicates that the proposed device is an excellent approach to avoid the problems caused by the differences of characteristics between membrane filters.
3.2 Selection of membrane filter type
To select a suitable membrane filter for enrichment of RhB, four commonly used membrane filters, i.e., mixed cellulose esters membrane filter, cellulose acetate membrane filter, nylon membrane filter and polytetrafluoroethylene (hydrophilic) membrane filter were tested. Several groups (n × 6) of sample solutions of 8 mL were filtered with different types of membrane filters using the developed six-channel device at vacuum degree of 0.098 MPa, respectively. The UV-vis diffuse reflectance spectra of each membrane filter concentrated RhB were measured. According to the results, there is scarcely any capacity of polytetrafluoroethylene membrane filter for enriching RhB, while mixed cellulose esters membrane filter provides the strongest absorbance and shows the most excellent enrichment efficiency compared with other two types of membrane filters. Therefore, mixed cellulose esters membrane filter was selected for subsequent experiments.3.3 Effect of pH
The variation of the pH of sample solutions can lead to the deprotonation or the protonation of a neutral molecule, which will further result in the change of solubility of analytes and the efficiency of the membrane filtration-enrichment procedure. To achieve an excellent enrichment performance, the pH should be adjusted to an appropriate value. The effect of sample solution pH was studied within the range of 1 to 11 adjusted by using HCl and NaOH solutions. As it is shown in Fig. 2, a slight variation on the absorbance is observed when the pH is in the range of 7–11, while a remarkable growth of the absorbance emerges when the pH is lower than 7. This may be explained by the fact that the deprotonation of the carboxyl of RhB cannot conduct under the acid condition, which decreases the solubility of RhB and makes it enriched more efficiently by the membrane filter. Taking the sensitivity into account, pH of 1 was chosen for the rest of the work. | ||
| Fig. 2 Effect of pH on absorbance of RhB at 554 nm. Experiment conditions: concentration of RhB, 30 μg L−1; sample volume, 18 mL; vacuum degree, 0.05 MPa. | ||
3.4 Effect of vacuum degree
The vacuum degree of a vacuum pump has an immediate impact on the membrane flux and the performance of interactions between analytes and the membrane filter, thus affecting the efficiency of filtration. The effect of vacuum degree was investigated within the range of 0.02 to 0.09 MPa. The results in Fig. 3 indicate that as the decline of vacuum degree, the maximum absorbance values of membrane filters concentrated RhB increase gradually, and the time for filtration also increases. Considering both the sensitivity and the operation time, the vacuum degree of 0.04 MPa was selected. | ||
| Fig. 3 Effect of vacuum degree on absorbance of RhB at 554 nm. Experiment conditions: concentration of RhB, 30 μg L−1; pH = 1; sample volume, 18 mL. | ||
3.5 Effect of sample volume
To obtain reliable and reproducible analytical results and high sensitivity, large volume of solution is usually needed. When selecting a suitable sample volume, both the adsorption capacity of the membrane filter and the operation time should be taken into account besides the sensitivity. Same sample solutions with different volumes in the range of 5–30 mL were filtered under constant conditions. The results listed in Fig. 4 show that there is a significant increase on the absorbance values when the sample volume is in the range of 5–20 mL, while the sample volume keeps increasing to 30 mL, the absorbance values remain about the same. Therefore, 20 mL of sample solution was adopted in further experiments.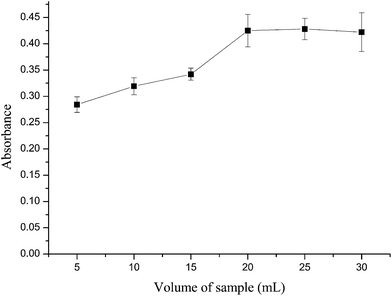 | ||
| Fig. 4 Effect of sample volume on absorbance of RhB at 554 nm. Experiment conditions: concentration of RhB, 30 μg L−1; pH = 1; vacuum degree, 0.04 MPa. | ||
3.6 Analytical performance of the method
Once optimum conditions have been confirmed, the analytical performance of the method was evaluated. The enrichment efficiency of the proposed membrane filtration-enrichment method was assessed with 30 μg L−1 RhB by LC-MS. The analytical results of LC-MS showed that the concentration of RhB in effluent solution is 0.4 μg L−1. Thus the enrichment efficiency was calculated to be (30 − 0.4)/30 = 98.7%.To investigate the quantitative relationship between c, RhB concentration in solution, and A and between F(R∞) and c, a series of experiments were conducted with RhB solutions in the concentration range of 2–30 μg L−1 and relevant values of maximum absorbance (at 554 nm) were measured and plotted in Fig. 5(a). From Fig. 5(a) it follows that linearity of calibration curve is achieved only at the range of 2–6 μg L−1 with the correlation coefficient (R2) of 0.9858 (inset of Fig. 5(a)). The limit of detection, defined as LOD = 3Sb/m (where Sb and m are standard deviation of the blank solution and slope of the calibration curve, respectively), is 0.12 μg L−1. However, in the case of the concentration range of 2–30 μg L−1, the relationship between the absorbance A and the concentration of RhB c is nonlinear. When c is fitted with A by eqn (6), it provides a good fitting with R2 of 0.9928. Then, F(R∞) is calculated by eqn (6) with A and plotted as a function of c in Fig. 5(b), it yields a linear curve passing through the origin with R2 of 0.9924, and this suggests that eqn (6) can be employed as a linear model for quantitative analysis in a relatively wide concentration range.
When directly measuring the UV-vis spectra of RhB solutions with a 1 cm cell, the maximum absorbance value of 50 μg L−1 RhB solution was only 0.0070, and the LOD was calculated to be 18.7 μg L−1.
Under the optimal conditions, the repeatability of the proposed method was evaluated using 30, 10 and 5 μg L−1 RhB solutions. For each concentration, three membrane filters were selected randomly to fulfil enrichment experiments and measured at every hole after the enrichment, thus with six absorbance values measured, RSDs of each membrane filter were calculated, respectively, and the average RSD of three membrane filters was used to evaluate the repeatability. The three average RSDs corresponding to the three concentration solutions were 3.6%, 3.8% and 3.8%, respectively.
It can be readily found that the proposed method provides a significant enhancement of the sensitivity (comparing with the conventional transmission UV-vis spectroscopy method) and repeatability (comparing with the conventional membrane filtration-enrichment UV-vis spectroscopy method).
3.7 Interference studies
To evaluate the selectivity of the proposed method, the effect of the potential interfering compounds which usually exist in real samples were investigated. Experiments were carried out with a series of 30 μg L−1 RhB solutions in the presence of various amounts of individual inorganic salts and two organic dyes, respectively. A given species is considered as interfering, when it causes a ±5% variation in the absorbance of the sample. The results obtained are illustrated in Table 2. The tolerance ratios of inorganic ions are within the limit of 500–3500. While malachite green and fluorescein can be tolerated within the ratios of 5.| Substance added | Tolerance ratio (interferent/analyte w/w) | Added as |
|---|---|---|
| K+ | 3500 | K2CO3, KNO3 |
| Na+ | 2000 | NaCl |
| Cl− | 2000 | CaCl2, MgCl2·6H2O |
| NO3− | 2000 | KNO3 |
| CO32− | 2000 | K2CO3 |
| SO42− | 1000 | CuSO4·5H2O |
| Ca2+ | 500 | CaCl2 |
| Ma2+ | 500 | MgCl2·6H2O |
| Cu2+ | 500 | CuSO4·5H2O |
| Malachite green | 5 | — |
| Fluorescein | 5 | — |
3.8 Real sample analysis
The presented multichannel membrane filtration-enrichment device was employed to quantify RhB in soft drink and river water samples to assess its accuracy and applicability. As a consequence of high contents of carbohydrates in drinks, which are usually macromolecular compounds and will occupy the pore space of the membrane filter, thereby influencing the filtration. The soft drink applied in this study was diluted by 6 times with ultra-pure water. All the samples were spiked with RhB at the concentrations of 5, 10 and 15 μg L−1, respectively. Recovery experiments were conducted and the results are listed in Table 3. It is found that the concentrations of RhB in the two real samples are below the LOD. The recoveries of the target analyte are in the acceptable range of 95.7–108.7% with the RSDs (n = 3) of 2.5–7.6%, which verifies the feasibility of the current method.4. Conclusion
In summary, we have developed a very simple but effective homemade multichannel device for the membrane filtration-enrichment process. The device is able to enrich six samples simultaneously on different positions of a membrane filter. With the support of the proposed device, the effects caused by the differences of characteristics between membrane filters could be conveniently avoided, and the performance of both the membrane filtration-enrichment technique and the subsequent on-line detection were significantly enhanced. The proposed approach was evaluated by enriching rhodamine B in aqueous samples as a case study. The main parameters influencing the filtration process were thoroughly optimized. The results reveal that the developed multichannel membrane filtration-enrichment device is efficient, reliable and reproducible and the applicability of it can be extended to a number of other analytes.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21205041) and Science and Technology Commission of Shanghai Municipality (13142201200).References
- D. Han and K. H. Row, Microchim. Acta, 2011, 176, 1–22 CrossRef.
- F. Pena-Pereira, I. Lavilla and C. Bendicho, Spectrochim. Acta, Part B, 2009, 64, 1–15 CrossRef PubMed.
- N. J. Wang, S. F. Mao, W. Liu, J. Wu, H. F. Li and J. M. Lin, RSC Adv., 2014, 4, 11919–11926 RSC.
- V. Andruch, L. Kocúrová, I. S. Balogh and J. Škrlíková, Microchem. J., 2012, 102, 1–10 CrossRef CAS PubMed.
- M. P. Arena, M. D. Porter and J. S. Fritz, Anal. Chim. Acta, 2003, 482, 197–207 CrossRef CAS.
- W. Li, X. Zhang, P. Tong, T. Wu, H. Hu, M. Wang and Y. Du, Spectrochim. Acta, Part A, 2014, 124, 159–164 CrossRef CAS PubMed.
- M. C. Hennion, J. Chromatogr. A, 1999, 856, 3–54 CrossRef CAS.
- V. Camel, Spectrochim. Acta, Part B, 2003, 58, 1177–1233 CrossRef.
- M. M. Tian, D. X. Chen, Y. L. Sun, Y. W. Yang and Q. Jia, RSC Adv., 2013, 3, 22111–22119 RSC.
- H. Lord and J. Pawliszyn, J. Chromatogr. A, 2000, 885, 153–193 CrossRef CAS.
- C. Dietz, J. Sanz and C. Camara, J. Chromatogr. A, 2006, 1103, 183–192 CrossRef CAS PubMed.
- T. Kumazawa, X. P. Lee, K. Sato and O. Suzuki, Anal. Chim. Acta, 2003, 492, 49–67 CrossRef CAS.
- I. Narin and M. Soylak, Anal. Chim. Acta, 2003, 493, 205–212 CrossRef CAS.
- J. Iqbal, C. Yin, J. Geng, X. Li, X. Li, Y. Du and B. X. Liu, Microchim. Acta, 2012, 177, 195–200 CrossRef CAS.
- C. H. Yin, J. Iqbal, H. L. Hu, B. X. Liu, L. Zhang, B. L. Zhu and Y. P. Du, J. Hazard. Mater., 2012, 233, 207–212 CrossRef PubMed.
- B. Dolgin, V. Bulatov, J. Japarov, E. Elish, E. Edri and I. Schechter, Talanta, 2010, 81, 1482–1488 CrossRef CAS PubMed.
- I. M. Raimundo and R. Narayanaswamy, Sens. Actuators, B, 2003, 90, 189–197 CrossRef CAS.
- U. Divrikli, A. A. Kartal, M. Soylak and L. Elci, J. Hazard. Mater., 2007, 145, 459–464 CrossRef CAS PubMed.
- M. Soylak, Y. E. Unsal, N. Kizil and A. Aydin, Food Chem. Toxicol., 2010, 48, 517–521 CrossRef CAS PubMed.
- Y. Takahashi, H. Kasai, H. Nakanishi and T. M. Suzuki, Angew. Chem., Int. Ed. Engl., 2006, 45, 913–916 CrossRef CAS PubMed.
- G. Chen, H. Hu, T. Wu, P. Tong, B. Liu, B. Zhu and Y. Du, Food Control, 2014, 35, 218–222 CrossRef CAS PubMed.
- Y. Chen, S. Wang and T. Zhou, Anal. Lett., 1998, 31, 1233–1245 CrossRef CAS.
- Z. A. Alothman, Y. E. Unsal, M. Habila, A. Shabaka, M. Tuzen and M. Soylak, Food Chem. Toxicol., 2012, 50, 2709–2713 CrossRef CAS PubMed.
- Z. A. Alothman, Y. E. Unsal, M. Habila, M. Tuzen and M. Soylak, Desalin. Water Treat., 2014, 1–9 CrossRef.
- L. Hejazi, D. E. Mohammadi, Y. Yamini and R. G. Brereton, Talanta, 2004, 62, 183–189 CrossRef.
- J. S. Fritz, M. P. Arena, S. A. Steiner and M. D. Porter, J. Chromatogr. A, 2003, 997, 41–50 CrossRef CAS.
- M. P. Arena, M. D. Porter and J. S. Fritz, Anal. Chem., 2002, 74, 185–190 CrossRef CAS.
- T. D. Brock, Membrane Filtration: A User's Guide and Reference Manual, Science Tech, 1983 Search PubMed.
- S. Chang, T. David Waite, A. I. Schäfer and A. G. Fane, Environ. Sci. Technol., 2003, 37, 3158–3163 CrossRef CAS.
- A. I. Schafer, I. Akanyeti and A. J. Semiao, Adv. Colloid Interface Sci., 2011, 164, 100–117 CrossRef PubMed.
- P. D. G. Kortüm, Reflectance Spectroscopy: Principles, Methods, Applications, Springer Berlin, Heidelberg, 1969, pp. 5–216 Search PubMed.
- P. Kubelka and F. Munk, Zeitschrift fur Technische Physik, 1931, 12, 596–601 Search PubMed.
| This journal is © The Royal Society of Chemistry 2014 |



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)