A triple bond side-chained 2D-conjugated benzodithiophene based photovoltaic polymer†
Shuguang Wen‡
b,
Xiao Yun‡ab,
Weichao Chenb,
Qian Liuab,
Dangqiang Zhub,
Chuantao Gub,
Mingliang Sun*a and
Renqiang Yang*b
aInstitute of Material Science and Engineering, Ocean University of China, Qingdao 266100, People's Republic of China. E-mail: mlsun@ouc.edu.cn; Fax: +86-532-66781927; Tel: +86-532-66781690
bCAS Key Laboratory of Bio-based Materials, Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, Qingdao 266101, People's Republic of China. E-mail: yangrq@qibebt.ac.cn; Fax: +86-532-80662778; Tel: +86-532-80662700
First published on 30th October 2014
Abstract
A series of triple bond side-chained benzodithiophene copolymers, derived from 4,8-bis(1-ethynyl-3,5-bis(octyloxy)phenyl)-benzo[1,2-b:4,5-b′]dithiophene, were synthesized by Stille coupling reactions. Several electron acceptors are introduced into the polymer backbone to tune the photo-electronic properties of the copolymers. The obtained copolymers are readily soluble in common organic solvents, such as toluene, THF and chloroform. The number average molecular weights of these polymers were determined by GPC using a polystyrene standard, ranging from 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 to 28
400 to 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 which are decent results for polymers with a rigid triple bond side chain. These polymers show high decomposition temperature (403–419 °C), narrow band gap (1.27–1.89 eV), and low-lying HOMO energy level (−5.4 to −5.6 eV). The best polymer solar cell (PSC) device based on the copolymer PBDTPA-TPD and PC61BM showed a power conversion efficiency (PCE) of 0.93% with a Voc = 0.96 V, Jsc = 1.9 mA cm−2, and FF = 51%, which is one of the highest Voc values achieved by a benzodithiophene polymer.
300 which are decent results for polymers with a rigid triple bond side chain. These polymers show high decomposition temperature (403–419 °C), narrow band gap (1.27–1.89 eV), and low-lying HOMO energy level (−5.4 to −5.6 eV). The best polymer solar cell (PSC) device based on the copolymer PBDTPA-TPD and PC61BM showed a power conversion efficiency (PCE) of 0.93% with a Voc = 0.96 V, Jsc = 1.9 mA cm−2, and FF = 51%, which is one of the highest Voc values achieved by a benzodithiophene polymer.
1. Introduction
Polymer solar cells (PSCs) have attracted increasing interest due to their light-weight, large-area, and flexibility through solution processing.1–4 PSCs usually adopt a bulk-heterojunction (BHJ) structure, and the photoactive layer consists of an interpenetrating network of π-conjugated polymer donors and soluble fullerene acceptors.5–7 Among the different polymer donor units, the benzodithiophene (BDT) unit shows good planarity due to its symmetrical molecular structure, meanwhile BDT polymer also exhibits high carrier mobility due to strong intermolecular π–π stacking interactions.8–10 A 4,8-dialkoxy substituted BDT based donor–acceptor (D–A) type conjugated polymer, has shown an excellent maximum power conversion efficiency (PCE) of 9.35% (ref. 11) which is the highest PCE for PSCs. However, modification and optimization of BDT based polymers are still hot topics for higher efficiency PSCs. For example, by replacing strong electron-donating alkoxy chains with a weak electron donor, the HOMO energy level of the polymer is reduced, which can increase the open circuit voltage (Voc) of PSCs devices.12 By the concept of two dimensional (2D) conjugated BDT, dialkyl-thiophene substituted BDT is synthesized, which to some extent reduces the HOMO energy level and extends the absorption spectrum of the polymer, compared to dialkoxy substituted BDT.13–17 Besides the thiophene side chain of BDT, other conjugated side chains, such as thiophene[3,2-b]thiophene, benzene etc.,18 have also been tried to build a more efficient BDT photovoltaic polymer.According to the sp hybrid in the hybrid orbital theory, the triple bond in the alkynyl group is a weak electron withdrawing group, meanwhile performs high rigidity which is good for intermolecular stacking.19 By introducing alkynyl group into BDT side chain, the HOMO energy level of polymer materials can be lowered, due to enhanced π–π stacking. PCE of (tri-isopropyl-silyl) acetylene based BDT polymer have reached 5.76%,20 but strong rigidity of triple bond leads to poor solubility of the polymer, which make it inconvenient for PSCs devices processing.
In this paper, to make more soluble alkynyl substituted BDT donor materials, we have designed and synthesized two-dimension conjugated unit of dialkoxy-phenylacetylene substituted BDT, 4,8-bis(1-ethynyl-3,5-bis(octyloxy)phenyl)-benzo[1,2-b:4,5-b′]dithiophene (Scheme 1). Copolymerized with the acceptor TPD, DPP and TT (Scheme 2), three BDT based copolymers are obtained, and their optical properties, electrical properties and photovoltaic properties are studied.
2. Experimental section
2.1. Materials
Unless otherwise stated, all reagents and starting materials were used as commercially purchased without further purification. The TPD, DPP and TT comonomers were purchased from Derthon Optoelectronic Materials Science Technology Co Ltd. Tetrahydrofuran (THF) and toluene were distilled from sodium with benzophenone as indicator and N,N-dimethylformamide (DMF) was distilled from CaH2 under argon atmosphere before use. All air and water sensitive reactions were performed under argon or nitrogen atmosphere.2.2. Materials characterization
The nuclear magnetic resonance (NMR) spectra were recorded on a BrukerAdvanceIII 600 (600 MHz) using CDCl3 as the solvent. UV-vis absorption spectra were measured with a Hitachi U-4100 spectrophotometer. The molecular weight and polydispersity were determined by gel permeation chromatography (GPC) analysis using an ELEOS System with polystyrenes as the reference standard and THF as an eluent. Thermogravimetric analysis (TGA) was performed on a TA-Q600 analyzer with a heating rate of 20 °C min−1 under nitrogen. Electrochemical cyclic voltammetry (CV) measurements were conducted on an electrochemistry workstation (CHI660D) with a glassy carbon as working electrode, a platinum wire as counter electrode and an Ag/AgCl as reference electrode under nitrogen in a solution of Bu4NPF6 (0.1 M).2.3. Device fabrication
Photovoltaic devices were fabricated on pre-patterned indium tin oxide (ITO) coated glass substrates with a layered structure of ITO/PEDOT:PSS/donor:PC61BM/Ca (10 nm)/Al (100 nm). The ITO coated glass substrates were cleaned in ultrasonic bath in acetone, toluene, methanol and isopropyl alcohol sequentially. After a twenty-minute oxygen plasma treatment, a thin layer of PEDOT:PSS (30 nm) was spin-coated onto the ITO anode and then dried at 120 °C for 20 min. The photosensitive layer was prepared by spin-coating a blend solution of the polymers and PC61BM with a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 in deoxygenated anhydrous o-dichlorobenzene on the ITO/PEDOT:PSS substrate and then annealed at 150 °C for 10 min in a glove box. 4% of DIO was added as processing additive to the blend solutions. The active layer thickness was around 90 nm. Finally, Ca (10 nm) and aluminum (100 nm) were thermally evaporated at a vacuum of ∼2 × 10−4 Pa on top of active layer. The photovoltaic performance was measured under illumination at 100 mW cm−2 AM 1.5G irradiation using a Xe arc lamp in an argon atmosphere (<0.1 ppm H2O and O2), and the current density–voltage (J–V) curves was obtained by Keithley 2400. The external quantum efficiency (EQE) was obtained by a source meter, silicon photodiode and a computer-controlled light source-monochromator-lock-in system.
1.5 in deoxygenated anhydrous o-dichlorobenzene on the ITO/PEDOT:PSS substrate and then annealed at 150 °C for 10 min in a glove box. 4% of DIO was added as processing additive to the blend solutions. The active layer thickness was around 90 nm. Finally, Ca (10 nm) and aluminum (100 nm) were thermally evaporated at a vacuum of ∼2 × 10−4 Pa on top of active layer. The photovoltaic performance was measured under illumination at 100 mW cm−2 AM 1.5G irradiation using a Xe arc lamp in an argon atmosphere (<0.1 ppm H2O and O2), and the current density–voltage (J–V) curves was obtained by Keithley 2400. The external quantum efficiency (EQE) was obtained by a source meter, silicon photodiode and a computer-controlled light source-monochromator-lock-in system.
2.4. Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) eluent) to obtain 1.3 g of light yellow powder (1.47 mmol, 45%). 1H NMR (CDCl3, 600 MHz), δ (ppm): 7.74 (d, 2H), 7.62 (d, 2H), 6.84 (d, 4H), 6.55 (t, 2H), 4.02 (t, 8H), 1.84 (m, 8H), 1.51 (m, 8H), 1.36 (m, 32H), 0.93 (t, 12H) (Fig. S4†).
1, v/v) eluent) to obtain 1.3 g of light yellow powder (1.47 mmol, 45%). 1H NMR (CDCl3, 600 MHz), δ (ppm): 7.74 (d, 2H), 7.62 (d, 2H), 6.84 (d, 4H), 6.55 (t, 2H), 4.02 (t, 8H), 1.84 (m, 8H), 1.51 (m, 8H), 1.36 (m, 32H), 0.93 (t, 12H) (Fig. S4†).3. Results and discussion
3.1. Synthesis and characterization
The general synthetic routes toward the monomer and copolymers are outlined in Schemes 1 and 2 respectively. All the compounds are characterized by 1H NMR spectra. They are in accordance with the literature21 for the precursors and show correctly the designed structure for polymers (see ESI Fig. S7–S9†). The obtained copolymers are readily soluble in common organic solvents, such as toluene, THF, and chloroform. The number-average molecular weights of these polymers were determined by GPC using a polystyrene standard, ranging from 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 to 28
400 to 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 with a polydispersity index (Mw/Mn) between 2.41 and 9.72. The molecular weights data is summarized in Table 1.
300 with a polydispersity index (Mw/Mn) between 2.41 and 9.72. The molecular weights data is summarized in Table 1.
| Polymer | Mn (k) | Mw (k) | PDI | λmax-S (nm) | λmax-F (nm) | ε (cm−1) thin film | Eoptg (eV) |
|---|---|---|---|---|---|---|---|
| a λmax-S: absorption peak in solution; λmax-F: absorption peak in film.b Eoptg: optical band gap is calculated from 1240/λonset. | |||||||
| PBDTPA-TPD | 23.9 | 57.7 | 2.41 | 587 | 613 | 4.1 × 104 | 1.89 |
| PBDTPA-TT | 15.4 | 81.2 | 5.25 | 603 | 624 | 2.4 × 104 | 1.37 |
| PBDTPA-DPP(OD) | 28.3 | 275.6 | 9.72 | 683 | 784 | 4.7 × 104 | 1.27 |
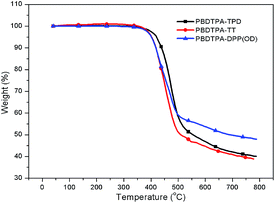
The thermal properties of the polymer were measured with a heating rate of 20 °C min−1 under inert atmosphere. As shown in Fig. 1, the decomposition temperature (Td) at 5% weight loss of PBDTPA-TPD, PBDTPA-TT and PBDTPA-DPP(OD) was 419.7 °C, 403.7 °C and 401.7 °C respectively. The high decomposition temperature decreases the possibility of the deformation of the polymer film morphology and the degradation of the polymer active layer under applied electric fields in solar cell devices.
3.2. Optical properties
The absorption spectra of the polymers were measured both in chloroform solution and in thin films (Fig. 2, Table 1). Since the different electron acceptors of copolymers lead to various absorption spectral features, PBDTPA-TPD, PBDTPA-TT and PBDTPA-DPP(OD) exhibit absorption peaks at ∼587, 603 and 683 nm respectively. Compared to the absorption in solution, the thin films show red-shifted UV-vis absorption spectrum, with absorption peaks at ∼613, 624 and 784 nm, respectively. Especially for PBDTPA-DPP(OD), the absorption spectrum becomes broader and shows a 101 nm red-shifted in thin film, which can be attributed to the planar polymer chain structure and effective inter-chain π–π stacking in the solid state. The introduction of alkynyl group enhances intermolecular interactions by extending the 2D conjugation of polymers. The absorption coefficients were tested in thin film and middle value (<5 × 104 cm−1) were obtained for all three polymers, which may cause limited photocurrent in solar cells.223.3. Electrochemical characterization
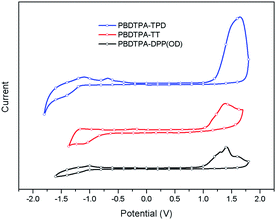
Cyclic voltammogram (CV) (Fig. 3) was performed to evaluate the HOMO and LUMO energy levels of all the polymers. HOMO and ELUMO were estimated from the onset oxidation potential and reduction potential of the polymer film.23 The HOMO and LUMO levels are calculated according to the empirical formula EHOMO = −e(Eonsetox + 4.4) eV and ELUMO = −e(Eonsetred + 4.4) eV. The data are listed in Table 2. The polymers show deep HOMO level around −5.6 and −5.4 eV, which may result in high open circuit voltage (Voc) in OSC device. Meanwhile, the LUMO levels were calculated from optical band gap. The materials of PBDTPA-TT and PBDTPA-DPP(OD) exhibit low LUMOopt value of below −4.0 eV, which may lead to insufficient exciton dissociation at donor–acceptor interfaces.243.4. Photovoltaic performance
Polymer solar cells (PSCs) devices were fabricated with the resultant polymer as the donor and PC61BM as the acceptor to investigate the photovoltaic properties of the polymer. The device structure is ITO/PEDOT:PSS/polymer:PC61BM/Ca/Al. To improve the photovoltaic performance of the device, 1,8-diiodooctane (DIO) was used as the additive and the optimized D/A ratio of polymer: PC61BM was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 (Fig. S10†). Fig. 4 shows the current density–voltage curves (J–V) of the devices under the illumination of AM 1.5G (100 mW cm−2). The PBDTPA-TPD/PC61BM device exhibited the best PCE of 0.93%, Voc of 0.96 V. Due to the low-lying HOMO energy level of PBDTPA-TPD (−5.4 eV), the PSCs based on PBDTPA-TPD show nearly 1 V (0.96 V) Voc which is one of the highest Voc value achieved by benzodithiophene polymer. For PBDTPA-DPP(OD)/PC61BM based PSCs devices, the best result was obtained with a PCE = 0.39% and a Voc of 0.45 V. PBDTPA-TT based device performances wasn't listed in Table 3, because it does not show photovoltaic response in the ITO/PEDOT:PSS/polymer:PC61BM/Ca/Al device structure. For polymers PBDTPA-DPP(OD) and PBDTPA-TT, the deep LUMO level (<−4.0 eV) may be a detrimental factor for the low PCE value, because the LUMO–LUMO offset with PC61BM acceptor is less than 0.3 eV and exciton dissociation could not be sufficiently efficient. Furthermore, low value of absorption coefficient would also reduce the PCE of OSC (Table 1). The dark J–V curves were tested for polymers of PBDTPA-TPD and PBDTPA-DPP(OD) (Fig. 4). It shows good diode character.
1.5 (Fig. S10†). Fig. 4 shows the current density–voltage curves (J–V) of the devices under the illumination of AM 1.5G (100 mW cm−2). The PBDTPA-TPD/PC61BM device exhibited the best PCE of 0.93%, Voc of 0.96 V. Due to the low-lying HOMO energy level of PBDTPA-TPD (−5.4 eV), the PSCs based on PBDTPA-TPD show nearly 1 V (0.96 V) Voc which is one of the highest Voc value achieved by benzodithiophene polymer. For PBDTPA-DPP(OD)/PC61BM based PSCs devices, the best result was obtained with a PCE = 0.39% and a Voc of 0.45 V. PBDTPA-TT based device performances wasn't listed in Table 3, because it does not show photovoltaic response in the ITO/PEDOT:PSS/polymer:PC61BM/Ca/Al device structure. For polymers PBDTPA-DPP(OD) and PBDTPA-TT, the deep LUMO level (<−4.0 eV) may be a detrimental factor for the low PCE value, because the LUMO–LUMO offset with PC61BM acceptor is less than 0.3 eV and exciton dissociation could not be sufficiently efficient. Furthermore, low value of absorption coefficient would also reduce the PCE of OSC (Table 1). The dark J–V curves were tested for polymers of PBDTPA-TPD and PBDTPA-DPP(OD) (Fig. 4). It shows good diode character.
 | ||
| Fig. 4 J–V curves of the PSCs based on the blend of PBDTPA-TPD or PBDTPA-DPP(OD)/PC61BM under the illumination of AM 1.5, 100 mW cm−2. | ||
| Active layer | Voc (V) | Jsc (mA cm−2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| PBDTPA-TPD/PC61BM | 0.96 | 1.90 | 51 | 0.93 |
| PBDTPA-DPP(OD)/PC61BM | 0.45 | 2.26 | 39 | 0.39 |
The external quantum efficiency (EQE) spectra of the devices are shown in Fig. 5. EQE value of <20% were obtained for polymers PBDTPA-TPD and PBDTPA-DPP(OD). The EQE spectrum of PBDTPA-DPP(OD) shows relatively low value beyond 500 nm, while its maximum absorption in film is at about 784 nm. This mismatch reflect insignificant contribution from the major absorption of PBDTPA-DPP(OD) to the photocurrent of solar cell.25 That is to say, the conversion of the low-energy excitons (from the polymer's absorption) to harvestable free charges is not efficient. This is most probably due to the poor dissociation efficiency of the low-energy excitons as the polymer-PC61BM LUMO energy difference is too small (<0.3 eV).26 This induces less-effective utilization of the absorption and low Jsc is obtained as a result.
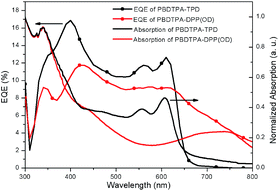 | ||
| Fig. 5 EQE curves of the PSCs based on the blend of PBDTPA-TPD or PBDTPA-DPP(OD)/PC61BM and optical absorptions for the corresponding polymer/PC61BM blend film. | ||
4. Conclusions
In summary, a series of triple bond side-chained benzodithiophene copolymer, derived from 4,8-bis(1-ethynyl-3,5-bis(octyloxy)phenyl)-benzo[1,2-b:4,5-b′]dithiophene, were synthesized. By introducing soluble bis(octyloxy)phenyl substituted triple band as side chain of DBT polymer, decent molecule weight (15.4 k to 28.3 k) is achieved for polymers with rigid triple band side chain. These polymers show high decomposition temperature and low-lying HOMO energy level. The best PSCs device based on the copolymer PBDTPA-TPD and PC61BM showed a nearly 1 V (0.96 V) Voc. Further work to make more efficiency triple bond side-chained benzodithiophene polymer is underway.Acknowledgements
The authors are deeply grateful to the National Natural Science Foundation of China (Project no. 21274134, 21202181, 51173199 and 61405209), New Century Excellent Talents in University (NCET-11-0473), and Qingdao Municipal Science and Technology Program (13-1-4-200-jch) for financial support.References
- B. C. Thompson and J. M. Fréchet, Angew. Chem., Int. Ed., 2008, 47, 58 CrossRef CAS PubMed.
- J. W. Chen and Y. Cao, Acc. Chem. Res., 2009, 42, 1709 CrossRef CAS PubMed.
- G. Li, R. Zhu and Y. Yang, Nat. Photonics, 2012, 6, 153 CrossRef CAS.
- R. Duan, L. Ye, X. Guo, Y. Huang, P. Wang, S. Zhang, J. Zhang, L. Huo and J. Hou, Macromolecules, 2012, 45, 3032 CrossRef CAS.
- G. Dennler, M. C. Scharber and C. J. Brabec, Adv. Mater., 2009, 21, 1323 CrossRef CAS.
- P. M. Beaujuge and J. M. Freìchet, J. Am. Chem. Soc., 2011, 133, 20009 CrossRef CAS PubMed.
- H. Zhou, L. Yang and W. You, Macromolecules, 2012, 45, 607 CrossRef CAS.
- H. Pan, Y. Li, Y. Wu, P. Liu, B. S. Ong, S. Zhu and G. Xu, J. Am. Chem. Soc., 2007, 129, 4112 CrossRef CAS PubMed.
- L. Huo and J. Hou, Polym. Chem., 2011, 2, 2453 RSC.
- J. Hou, M. H. Park, S. Zhang, Y. Yao, L. M. Chen, J. H. Li and Y. Yang, Macromolecules, 2008, 41, 6012 CrossRef CAS.
- S. H. Liao, Y. S. Cheng and S. A. Chen, Adv. Mater., 2013, 25, 4766 CrossRef CAS PubMed.
- D. Lee, E. Hubijar, G. J. D. Kalaw and J. P. Ferraris, Chem. Mater., 2012, 24, 2534 CrossRef CAS.
- J. Min, Z. G. Zhang, S. Y. Zhang and Y. F. Li, Chem. Mater., 2012, 24, 3247 CrossRef CAS.
- Y. Huang, X. Guo, F. Liu, L. Huo, Y. Chen, T. P. Russell, C. C. Han, Y. F. Li and J. H. Hou, Adv. Mater., 2012, 24, 3383 CrossRef CAS PubMed.
- L. Dou, W. H. Chang, J. Gao, C. C. Chen, J. B. You and Y. Yang, Adv. Mater., 2012, 25, 825 CrossRef PubMed.
- J. Y. Yuan, Z. C. Zhai, H. L. Dong, J. Li, Z. Q. Jiang, Y. F. Li and W. L. Ma, Adv. Funct. Mater., 2012, 23, 885 CrossRef.
- M. J. Zhang, Y. Gu, X. Guo, F. Liu, S. Q. Zhang, L. J. Huo, T. P. Russell and J. H. Hou, Adv. Mater., 2013, 25, 4944 CrossRef CAS PubMed.
- J. H. Kim, C. E. Song, B. S. Kim, I. N. Kang, W. S. Shin and D. H. Hwang, Chem. Mater., 2014, 26, 1234 CrossRef CAS.
- P. Sista, H. Nguyen, J. W. Murphy, J. Hao, D. K. Dei, K. Palaniappan, J. Servello, R. S. Kularatne, B. E. Gnade, B. Xue, P. C. Dastoor, M. C. Biewer and M. C. Stefan, Macromolecules, 2010, 43, 8063 CrossRef CAS.
- C. Bathula, S. E. Cho, S. Badgujar, S. J. Hong, I. N. Kang, S. J. Moon, J. Lee, S. Cho, H. K. Shim and S. K. Lee, J. Mater. Chem., 2012, 22, 22224 RSC.
- H. G. Kim, S. B. Jo, C. Shim, J. Lee, J. Shin, E. C. Cho, S. G. Ihn, Y. S. Choi, Y. Kim and K. Cho, J. Mater. Chem., 2012, 22, 17709 RSC.
- B. C. Thompson and J. M. J. Fréchet, Angew. Chem., Int. Ed., 2008, 47, 58 CrossRef CAS PubMed.
- Y. F. Li, Y. Cao, J. Gao, D. L. Wang, G. Yu and A. J. Heeger, Synth. Met., 1999, 99, 243 CrossRef CAS.
- Y. Li, B. Xu, H. Li, W. Cheng, L. Xue, F. Chen, H. Lu and W. Tian, J. Phys. Chem. C, 2011, 115, 2386 CAS.
- M. Zhang, X. Guo, X. Wang, H. Wang and Y. Li, Chem. Mater., 2011, 23, 4264 CrossRef CAS.
- M. C. Scharber and N. S. Sariciftci, Prog. Polym. Sci., 2013, 38, 1929 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra10154e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |