Three 3D coordination polymers based on [1,1′:4′,1′′-terphenyl]-2′,4,4′′,5′-tetracarboxylate demonstrating magnetic properties and selective sensing of Al3+/Fe3+over mixed ions†
Bei-Bei Kang,
Na Wei and
Zheng-Bo Han*
College of Chemistry, Liaoning University, Shenyang, 110036, P. R. China. E-mail: ceshzb@lnu.edu.cn
First published on 12th November 2014
Abstract
Three three-dimensional (3D) coordination polymers with the formulae [Cd(TPTC)0.5(H2O)2] ·DMA (1), [Co2(TPTC)(H2O)1.5(CH3OH)0.5] (2), and [Mn2(TPTC)(H2O)(DMA)]·DMA (3) (TPTC = [1,1′:4′,1′′-terphenyl]-2′,4,4′′,5′-tetracarboxylate acid) were solvothermally synthesized. They were characterized by thermogravimetric analyses, IR spectroscopy, X-ray powder diffraction, and single crystal X-ray diffraction. 1 features a 3D porous coordination polymer formed from Cd-carboxylate chains and the TPTC bridges. In 2 and 3, the uniform metal–carboxylate chains with triple bridges, (μ-EO-H2O)(μ-syn,syn-COO)2 and (μ-EO-H2O/DMA)(μ-syn,syn-COO)2 (EO = end-on), were extended by the TPTC arms. Complex 1 displayed strong fluorescent emission in the visible region. Interestingly, the emission intensities of 1 were increased upon the addition of Al3+ and quenched upon the addition of Fe3+, even in mixtures of ions. Thus, 1 can act as a useful material for sensing Fe3+ and Al3+ ions. The magnetic studies of 2 and 3 show that antiferromagnetic interactions between the Co(II) and Mn(II) centers exist.
Introduction
Metal–organic coordination polymers or metal–organic frameworks (MOFs) have become an increasingly promising area, for their esthetically pleasing crystalline structures as well as their enormous practical applications, such as gas adsorption and separation,1 catalysis,2 luminescence,3 electrical conductivity,4 and magnetism.5 A number of luminescent materials for sensing small molecules or metal ions are widely used in many areas, such as luminescent probes in biomedical assays and time-resolved microscopy, fluorescent lighting, and luminescent probes for chemical species,6 which have made MOF chemosensors a hot research topic.7 MOFs offer high photoluminescence efficiency, based on components, antennae effects, adsorbate-based emission and sensitization, and surface functionalization.8 Recently, studies of luminescent MOFs for sensing metal ions have developed significantly, and some successful fluorescent probes have been employed to determine the concentration of metal ions such as Ca2+, Cd2+ and Fe3+.9The design and construction of functional porous MOFs with magnetic properties is a challenge because it is difficult to obtain large pore sizes and relatively strong magnetic interactions simultaneously.10 Magnetic superexchange requires relatively short exchange pathways between two adjacent metal centers. The most popular synthetic strategy uses magnetic chains or clusters connected by longer organic linkers to form a porous open framework.11
Organic carboxylate ligands have been widely used in synthesizing MOFs with esthetic structures and unusual properties because of the controllable length of ligand and the various coordination modes of the carboxyl group.12 Herein, we use a new quadrangular ligand, H4TPTC, [1,1′:4′,1′′-terphenyl]-2′,4,4′′,5′-tetracarboxylic acid. We present a study of three 3D porous MOFs, [Cd(TPTC)0.5(H2O)2]·DMA (1), [Co2(TPTC)(H2O)1.5(CH3OH)0.5] (2), and [Mn2(TPTC)(H2O)(DMA)]·DMA (3), which were generated by changing only the metal salt. More interestingly, 1 exhibits Al3+/Fe3+-modulated fluorescence over mixed ions; 2 and 3 also show antiferromagnetic interactions between metal ions.
Experimental section
Materials and methods
All chemicals were commercially available and used without further purification. The C, H, and N microanalyses were carried out with a PerkinElmer 240 elemental analyzer. The FT-IR spectra were recorded from KBr pellets in the 4000–400 cm−1 range on a Nicolet 5DX spectrometer. Thermogravimetric analyses (TGA) were performed on a PerkinElmer Pyrisl (20–800 °C, 10 °C min−1, N2 gas flow). X-ray powder diffraction was recorded with a Bruker AXS D8 advanced automated diffractometer with Cu-Kα radiation. Luminescence spectra for the solid samples and liquid samples were taken with a Hitachi F-4500 fluorescence spectrophotometer and a Varian Cary Eclipse Fluorescence spectrophotometer, respectively. The magnetic data were collected on a Quantum Design MPMS SQUID-XL-5 magnetometer.Solvothermal synthesis
X-ray crystallography
Crystallographic data of all the complexes were collected at 173 K with an Apex II diffractometer with Mo-Kα radiation (λ = 0.71073 Å) and graphite monochromator using the ω-scan mode. The structure was solved by direct methods and refined on F2 by full-matrix least squares using SHELXTL.13 In 2, DMA molecules were highly disordered, and attempts to locate and refine the peaks were unsuccessful. The DMA molecules were removed from the data set using the SQUEEZE routine of PLATON14 and refined further using the data generated. Crystallographic data and experimental details for structural analyses are shown in Table 1. The CCDC reference number is 1013700–1013702 for 1–3.†| 1 | 2 | 3 | |
|---|---|---|---|
| a R1 = ∑||Fo|−|Fc||/∑|Fo|; wR2 = ∑[w(Fo2 − Fc2)2]/∑[w(Fo2)2]1/2. | |||
| Empirical formula | C15H18CdNO7 | C22.5H15Co2O10 | C30H28Mn2N2O11 |
| Formula weight | 436.70 | 563.21 | 702.42 |
| Wavelength/Å | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/c | C2/m | P21/n |
| a/Å | 8.7173(6) | 18.7009(15) | 12.6852(11) |
| b/Å | 19.4288(17) | 7.2352(5) | 14.7613(15) |
| c/Å | 9.6558(8) | 12.5595(9) | 16.0030(15) |
| β/° | 97.170(3) | 123.488(2) | 98.210(3) |
| V/Å−3 | 1622.6(2) | 1417.27(18) | 2965.9(5) |
| Z | 4 | 2 | 4 |
| Dc/mg m−3 | 1.788 | 1.320 | 1.573 |
| μ/mm−1 | 1.383 | 1.215 | 0.917 |
| F(000) | 876 | 568 | 1440 |
| Range for data collection/° | 2.99–25.00 | 3.10–26.38 | 3.05–26.38 |
| Reflections collected | 2800 | 5869 | 17987 |
| Max., min. transmission | 0.7694, 0.7064 | 0.7775, 0.6633 | 0.8052, 0.7290 |
| T/K | 173(2) | 173(2) | 173(2) |
| Data/restraints/parameters | 2800/24/225 | 1516/60/116 | 5467/18/417 |
| Final R indices [I > 2σ(I)]a | R1 = 0.0479, wR2 = 0.0782 | R1 = 0.0849, wR2 = 0.2254 | R1 = 0.0503, wR2 = 0.0886 |
| R indices (all data) | R1 = 0.0767, wR2 = 0.0859 | R1 = 0.1206, wR2 = 0.2481 | R1 = 0.1394, wR2 = 0.1136 |
| Largest diff. peak and hole/e Å−3 | 0.891, −0.860 | 1.417, −0.951 | 0.546, −0.442 |
Results and discussion
Crystal structures
Single-crystal X-ray structure analysis reveals that 1 crystallizes in the monoclinic space group P21/c, showing a 3D non-interpenetrated framework. The asymmetric unit of 1 contains one crystallographically independent Cd(II) center, a half TPTC ligand, two aqua ligands, and a disordered DMA molecule. As shown in Fig. 1a and b, the TPTC ligand coordinates to six Cd(II) ions via its four carboxylate groups, two of which adopt chelating mode and the other two adopt monodentate coordination modes. The central Cd(II) ion is coordinated by four oxygen atoms of the TPTC ligands and two aqua ligands. Thus, the central Cd(II) ions are connected by TPTC ligands through their two η1:η1 chelating and two syn–anti-μ2-η1:η1 carboxylate groups to generate a 2D layer (Fig. 1c). The layers are connected by the carboxylate groups of the TPTC ligands to form a 3D framework with channel dimensions of 8.212 × 7.124 Å2 (including the van der Waals radii of the atoms), and neighboring metal ions are linked by the haploid syn–anti-COO group, which induces a 1D uniform Cd-carboxylate chain running along the a-axis, with a Cd–Cd separation of 4.889 Å (Fig. 1d).In contrast to 1, complex 2 crystallizes in the monoclinic space group C2/m. There is 0.5 of a Co(II) ion, 0.25 of a fully-deprotonated TPTC ligand, 0.375 of an μ2-aqua ligand, and 0.125 of a CH3OH molecule in the asymmetric unit of 2. The CH3OH molecule may come from an impurity in the DMF solvent. As shown in Fig. 2a and b, each TPTC ligand connects eight Co(II) ions and each Co(II) links to four ligands. The coordination geometry around the Co(II) center can be described as a distorted octahedron with O–Co–O bond angles ranging from 84.9(3) to 180.0(2), and Co–O bond lengths ranging from 2.051(5) to 2.172(4) Å. The carboxylate groups of TPTC exhibit μ2-η1:η1 bridging and a syn–syn coordination configuration, and neighboring metal ions are linked by the triple (μ-EO-H2O)(μ-syn,syn-COO)2 bridges, with Co–Co = 3.6176(2) Å, which induces a 1D uniform Co-carboxylate chain running along the c-axis (Fig. 2c). The four carboxylate groups in the TPTC ligand separate the metal ions from different chains by 9.350 Å and 10.751 Å.
The single-crystal X-ray diffraction analysis reveals that complex 3 crystallizes in a monoclinic P21/n space group. The asymmetric unit of 3 is composed of three crystallographically independent Mn(II) centers, one fully-deprotonated TPTC ligand, one μ2-aqua ligand, one μ2-DMA molecule, and one free DMA molecule. As shown in Fig. 3a and b, the three types of Mn are all six-coordinated with distorted octahedral geometry, with four carboxylate O atoms from four different TPTC ligands, and also with two O atoms from one aqua and one terminally coordinated DMA ligand for Mn1, two DMA ligands for Mn2, and two aqua ligands for Mn3, respectively. Similar to 2, the carboxylate groups of the TPTC adopt a syn–syn coordination configuration and neighboring metal ions are linked by the triple (μ-EO-H2O/DMA)(μ-syn,syn-COO)2 bridges. Thus, 3 is built on a 1D Mn carboxylate chains and TPTC bridges (Fig. 3c) and is connected further by the TPTC ligands, which induces a large 1D irregular quadrangular channel with dimensions of approximately 9.944 × 6.286 Å2 (including the van der Waals radii of the atoms) (Fig. 3d).
PXRD patterns and thermogravimetric analysis
The simulated and experimental XRD patterns of the three compounds are shown in Fig. S1–S3.† Their peak positions are in good agreement with each other, confirming the purity of the synthesized bulk materials. The TGA curve (Fig. S4†) of 1 shows the first weight loss of 12.1% at ca. 275 °C, corresponding to the release of free DMA molecules (calcd 19.9%). For complex 2 (Fig. S5†), the first weight decrease of 27.0% appears in the range of 163 to 235 °C, which is consistent with the loss of coordinated water molecules and two lattice DMA molecules (calcd 26.9%). The second loss of 52.3% between 360 and 390 °C implies sudden decomposition, which corresponds to the collapse of the framework. The TGA curve (Fig. S6†) of 3 displays a decrease of 26.16% from 170 to 240 °C, coinciding with the loss of the aqua ligands and DMA molecules (calcd 27.3%), and then the framework collapse upon further heating.Photoluminescence properties
Previous studies have shown that d10 coordination polymers containing zinc may exhibit photoluminescence properties.15 The luminescence properties of 1 were investigated. As expected, 1 exhibits extraordinary photoluminescence behavior in the solid state (Fig. S7†) and in a methanol suspension. In the solid state, a strong fluorescent emission band at 387 nm was observed at room temperature, excited at 274 nm, and in methanol solution, a fluorescent emission band at 390 nm was observed at room temperature, excited at 250 nm. 1 shows a weaker emission band blue-shifted to different extents compared with the free H4TPTC ligand (Fig. S8†), which probably originates from the ligand-to-metal charge transfer (LMCT).16Based on the XRD patterns (Fig. S9†), complex 1 retains its framework after immersion in methanol solutions containing different metal ions. The effects of a variety of relevant cations K+, Na+, Ag+, Ca2+, Cd2+, Co2+, Cu2+, Hg2+, Ni2+, Zn2+, Al3+, Cr3+, Gd3+, In3+ and Fe3+ on the fluorescent intensities of 1 were investigated, and the luminescence properties are shown in Fig. 4. The results indicate that the emission intensity of 1 increased significantly upon addition of Al3+ and the emission intensity of 1 was almost quenched upon addition of Fe3+. The highest peak at 390 nm is at least twice as intense as the corresponding band in the solution without Al3+. However, the introduction of other metal ions either did not affect or weakened the intensity.
 | ||
| Fig. 4 Room-temperature luminescent intensity of 1 at 390 nm in methanol suspension upon addition of various metal ions (excited at 250 nm). | ||
The luminescent intensity of Mn+ relies on the efficiency of the energy transfer from the ligand to the Mn+ center.17 Sun et al. prepared [H2N(CH3)2][Eu(H2O)2(BTMIPA)]·2H2O, with [H2N(CH3)2]+ located in the channels, which can be replaced by Fe3+ and Al3+, which has a significant effect on the luminescence emissions.18a Similar to the reported metal-sensitive luminescent metal probes, the change of luminescent intensity in our investigation may result from the more effective intramolecular energy transfer process from the TPTC ligand to the Cd(II) with the addition of Al3+, whereas with the additional Fe3+, this energy transfer process is less effective.
To explore the fluorescence quenching and enhancement further, detailed studies of the luminescence properties of 1 in the presence of Fe3+ and Al3+ ions were carried out. Concentration-dependent luminescence measurements were also carried out (Fig. S10a and b†). However, the PL intensities for Fe3+- and Al3+-loaded samples changed dramatically when the concentration was increased. For the Fe3+-loaded sample, the PL intensity decreased gradually, and when the concentration reached 10−3 mol L−1, the luminescence was quenched completely. For the Al3+-loaded sample, the PL intensity increased gradually and reached a maximum at a concentration of 9 × 10−4 mol L−1, and after that, the intensity decreased gradually. Based on these results, we propose the following mechanism. First, it is possible for the Fe3+ and Al3+ ions to bind to the inner surface of the channels. In the structure of 1, the carboxylic oxygen atoms inside the channel, the coordinated water molecules located on Cd2+, and the water molecules in the channel can create a favorable coordination environment for metal ions. Second, the exchange between Fe3+/Al3+ and Cd2+ may collapse the framework of 1.18
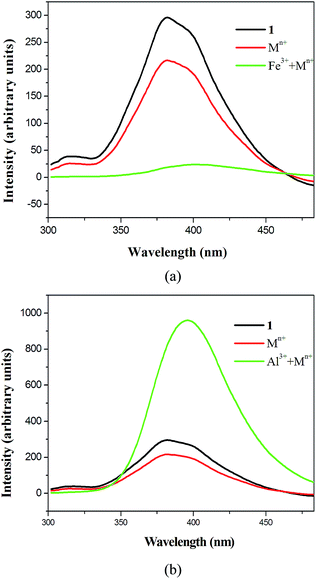
To confirm the high selectivity for Al3+ and Fe3+ ions over other metal ions, we studied the luminescence of 1 in a methanol solution containing mixed metal ions (Na+, Hg2+, Mg2+, Ni2+, Zn2+). The emission spectrum of the mixed-ion-loaded methanol solution decreased significantly, compared with the original one, as shown in Fig. 5a. However, when 1 was immersed in a methanol solution containing mixed metal ions including Fe3+ ions, the luminescence of 1 was quenched, indicating that the presence of other metal ions did not interfere with selectivity for Fe3+ ions. The detection of Al3+ ions over other metal ions was also examined and the emission spectrum shows that the intensity decreased significantly when Al3+ ions were absent, and the luminescence increased when Al3+ ions were present, as shown in Fig. 5b. At low Al3+/Fe3+ concentrations in mixed metal ion solutions, 1 is also highly sensitive to Al3+/Fe3+ ions (Fig. S10†). Thus, 1 is a good material for the selective sensing of Fe3+ and Al3+ ions. The high sensitivity of 1 for Al3+/Fe3+ ions indicates the promise of this type of luminescent material for sensing ions in biological systems.19
Magnetic properties
Magnetic measurements were performed for crystalline samples of complexes 2 and 3 in the temperature range of 2–300 K at 1 kOe, and the χM and χMT versus T curves are shown in Fig. 6. The two compounds are actually 3D porous frameworks, although in terms of magnetism, they can be considered as linear Co-(μ2-O)-Co and Mn-(μ2-O)-Mn chains, linked by the long carboxylate bridging ligands. | ||
| Fig. 6 Temperature dependence of χMT and χM under an applied field of 1 kOe for 2 (a) and 3 (b). The inset shows the χM−1 vs. T plot. | ||
For complex 2, the χM increased with decreasing temperature, reaching a maximum of 0.04 cm3 mol−1 at around 2.19 K. The χMT value per two Co(II) units at 300 K was 2.58 cm3 K mol−1, which is lower than the spin-only value of 3.75 cm3 K mol−1 expected for two magnetically isolated Co(II) ions (S = 3/2, g = 2.0).20 Decreasing the temperature decreased χMT gradually by a small amount from 2.58 cm3 K mol−1 at 300 to ca. 80 K, then a little more steeply, reaching 0.76 cm3 K mol−1 at 2.0 K, suggesting a dominant antiferromagnetic interaction between Co(II) centres.21 The inverse susceptibility plot as a function of temperature was linear above 100 K, following the Curie–Weiss law with a Weiss constant of θ = −42.22 K, and a Curie constant of C = 0.74 cm3 K mol−1. The negative Weiss constant indicates that there is a predominantly antiferromagnetic interaction between two adjacent Co(II) centres. However, there was a jump in the χMT vs. T plot at ca. 250 K that cannot be explained by the current model. This cannot be caused by a structural transition because the crystal of 2 remained unchanged down to 100 K. Other effects beyond structural transformations have not yet been found.22
For complex 3, χM increased with decreasing temperature, reaching a maximum of 0.02 cm3 mol−1 at around 2.09 K. The χMT value per Mn(II) unit at 300 K was 6.72 cm3 K mol−1, which is much lower than the spin-only value of 13.12 cm3 K mol−1 expected for three magnetically isolated Mn(II) ions (S = 5/2, g = 2.0). Decreasing the temperature decreased the χMT gradually by a small amount from 6.72 cm3 K mol−1 at 300 to ca. 80 K, then a little more steeply, reaching 0.43 cm3 K mol−1 at 2.0 K, suggesting a diamagnetic ground state between Mn(II) centres.23 The inverse susceptibility plot as a function of temperature was linear above 100 K, following the Curie–Weiss law with a Weiss constant of θ = −7.32 K, and a Curie constant of C = 0.23 cm3 K mol−1. The negative Weiss constant indicates that there is a predominantly antiferromagnetic interaction between two adjacent Mn(II) centres.
Conclusions
In summary, three 3D coordination polymers were constructed from rigid quadrangular ligands and different metal ions under the same reaction conditions. Polymers 1–3 exhibit different structures based on metal–carboxylate chains incorporating the TPTC ligand, and mean that the materials have excellent properties. 1 exhibits strong luminescence emissions in the solid state and in a methanol suspension at room temperature. The luminescence of 1 displayed high selectivity for Al3+ and Fe3+ ions, suggesting that it may be used as a luminescent probe for Al3+ or Fe3+. Magnetic studies of 2 and 3 showed that there are antiferromagnetic interactions between the metal centers.Acknowledgements
This work was granted financial support from National Natural Science Foundation of China (Grant 20871063, 21271096), Liaoning BaiQian Wan Talents (2010921066), the Program for Liaoning Excellent Talents in University (LR2011001), the Liaoning Natural Science Foundation, China (201102079), the Innovative Team Project of Department of Education of Liaoning Province, China (LT2011001), and the Liaoning University 211-Projects of the third period.References
- (a) O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae and M. Eddaoudi, J. Nat., 2003, 423, 705 CrossRef CAS PubMed; (b) J.-P. Zhang, Y.-B. Zhang, J.-B. Lin and X.-M. Chen, Chem. Rev., 2012, 112, 1001 CrossRef CAS PubMed; (c) J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477 RSC.
- (a) S. K. Ghosh, J. P. Zhang and S. Kitagawa, Angew. Chem., Int. Ed., 2007, 46, 7965 CrossRef CAS PubMed; (b) S. K. Ghosh, S. Bureekaew and S. Kitagawa, Angew. Chem., Int. Ed., 2008, 47, 3403 CrossRef CAS PubMed; (c) L. Ma, C. Abney and W. Lin, Chem. Soc. Rev., 2009, 38, 1248 RSC.
- (a) Y. Cui, Y. Yue, G. Qian and B. Chen, Chem. Rev., 2012, 112, 1126 CrossRef CAS PubMed; (b) C. Wang, O. Volotskova, K. Lu, M. Ahmad, C. Sun, L. Xing and W. Lin, J. Am. Chem. Soc., 2014, 17, 6171 CrossRef PubMed; (c) L. Wang, Y.-A. Li, F. Yang, Q.-K. Liu, J.-P. Ma and Y.-B. Dong, Inorg. Chem., 2014, 53, 9087 CrossRef CAS PubMed.
- (a) M.-H. Zeng, Q.-X. Wang, Y.-X. Tan, S. Hu, H.-X. Zhao, L.-S. Long and M. Kurmoo, J. Am. Chem. Soc., 2010, 132, 2561 CrossRef CAS PubMed; (b) A. A. Talin, A. Centrone, M. E. Foster, V. Stavila, P. Haney, R. A. Kinney, V. Szalai, F. El Gabaly and H. P. Yoon, Science, 2014, 6616, 66 CrossRef PubMed.
- (a) J. A. Real, A. B. Gaspar and M. C. Munoz, Dalton Trans., 2005, 2062 RSC; (b) C. J. Kepert, Chem. Commun., 2006, 695 RSC; (c) M. Kurmoo, Chem. Soc. Rev., 2009, 38, 1353 RSC; (d) A. Bousseksou, G. Molnar, L. Salmon and W. Nicolazzi, Chem. Soc. Rev., 2011, 40, 3313 RSC.
- (a) N. Sabbatini, M. Guardigli and J.-M. Lehn, Coord. Chem. Rev., 1993, 123, 201 CrossRef CAS; (b) A. W. Czarnik, Acc. Chem. Res., 1994, 27, 302 CrossRef CAS; (c) D. Parker, P. K. Senanayake and J. A. G. Williams, J. Chem. Soc., Perkin Trans. 2, 1998, 2129 RSC; (d) J. Cui, Z. Lu, Y. Li, Z. Guo and H. Zheng, Chem. Commun., 2012, 48, 7967 RSC; (e) B. Gole, A. K. Bar and P. S. Mukherjee, Chem. Commun., 2011, 47, 12137 RSC; (f) S.-R. Zhang, D.-Y. Du, J.-S. Qin, S.-L. Li, W.-W. He, Y.-Q. Lan and Z.-M. Su, J. Am. Chem. Soc., 2014, 53, 8105 CAS.
- (a) W. Liu, T. Jiao, Y. Li, Q. Liu, M. Tan, H. Wang and L. Wang, J. Am. Chem. Soc., 2004, 126, 2280 CrossRef CAS PubMed; (b) B. Zhao, X.-Y. Chen, P. Cheng, D.-Z. Liao, S.-P. Yan and Z.-H. Jiang, J. Am. Chem. Soc., 2004, 126, 15394 CrossRef CAS PubMed; (c) B. Gole, A. K. Bar and P. S. Mukherjee, Chem. Commun., 2011, 47, 12137 RSC.
- M. D. Allendorf, C. A. Bauer, R. K. Bhakta and R. J. T. Houk, Chem. Soc. Rev., 2009, 38, 1294 RSC.
- (a) J. Rocha, L. D. Carlos, F. A. A. Paz and D. Ananias, Chem. Soc. Rev., 2011, 40, 926 RSC; (b) W.-G. Lu, L. Jiang, X.-L. Feng and T.-B. Lu, Inorg. Chem., 2010, 48, 6997 CrossRef PubMed; (c) N. Wei, Y.-R. Zhang and Z.-B. Han, CrystEngComm, 2013, 15, 8883 RSC.
- (a) C. J. Kepert, Chem. Commun., 2006, 695 RSC; (b) G. Férey, Nat. Mater., 2003, 2, 136 CrossRef PubMed.
- (a) N. Masciocchi, S. Galli, V. Colombo, A. Maspero, G. Palmisano, B. Seyyedi, C. Lamberti and S. Bordiga, J. Am. Chem. Soc., 2010, 132, 7902 CrossRef CAS PubMed; (b) Z. B. Han, G. X. Zhang, M. H. Zeng, C. H. Ge, X. H. Zou and G. X. Han, CrystEngComm, 2009, 11, 2629 RSC.
- (a) Z.-B. Han, G.-X. Zhang, M.-H. Zeng, D.-Q. Yuan, Q.-R. Fang, J.-R. Li, J. Ribas and H.-C. Zhou, Inorg. Chem., 2010, 49, 769 CrossRef CAS PubMed; (b) Z.-B. Han, J.-W. Ji, H.-Y. An, W. Zhang, G.-X. Han, G.-X. Zhang and L.-G. Yang, Dalton Trans., 2009, 9807 RSC.
- G. M. Sheldrick, SHELXTL, Version 6.10, Bruker Analytical X-ray Systems, Madison, WI, USA, 2001 Search PubMed.
- A. L. Spek, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2009, 65, 148 CrossRef CAS PubMed.
- M. D. Allendorf, C. A. Bauer, R. K. Bhakta and R. J. T. Hou, Chem. Soc. Rev., 2009, 38, 1330 RSC.
- (a) J. Li, C. C. Ji, L. F. Huang, Y. Z. Li and H. G. Zheng, Inorg. Chim. Acta, 2011, 371, 33 CrossRef PubMed; (b) X. Q. Liu, Y. Y. Liu, Y. J. Hao, X. J. Yang and B. Wu, Inorg. Chem. Commun., 2010, 13, 513 Search PubMed; (c) E.-C. Yang, J. Li, B. Ding, Q.-Q. Liang, X.-G. Wang and X.-J. Zhao, CrystEngComm, 2008, 10, 158 RSC; (d) W.-G. Lu, L. Jiang, X.-L. Long and T.-B. Lu, Cryst. Growth Des., 2006, 6(2), 564 CrossRef CAS.
- N. Arnaud, E. Vaquer and J. Georges, Analyst, 1998, 123, 261 RSC.
- (a) Z. Chen, Y. Sun, L. Zhang, D. Sun, F. Liu, Q. Meng, R. Wang and D. Sun, Chem. Commun., 2013, 49, 11557 RSC; (b) E. Oliveira, C. Nuñez, B. Rodríguez-González, J. L. Capelo and C. Lodeiro, Inorg. Chem., 2011, 50(18), 8797 CrossRef CAS PubMed; (c) J. An, C. M. Shade, D. A. Chengelis-Czegan, S. Petoud and N. L. Rosi, J. Am. Chem. Soc., 2011, 133, 1220 CrossRef CAS PubMed; (d) M. Zheng, H. Q. Tan, Z. G. Xie, L. G. Zhang, X. B. Jing and Z. C. Sun, ACS Appl. Mater. Interfaces, 2013, 5, 1078 CrossRef CAS PubMed.
- (a) W. S. Liu, T. Q. Jiao, Y. Z. Li, Q. Z. Liu, M. Y. Tan, H. Wang and L. F. Wang, J. Am. Chem. Soc., 2004, 126, 2280 CrossRef CAS PubMed; (b) N. Wei, M.-Y. Zhang, X.-N. Zhang, G.-M. Li, X.-D. Zhang and Z.-B. Han, Cryst. Growth Des., 2014, 14(6), 3002 CrossRef CAS.
- M. J. Hossain, M. Yamasaki, M. Mikuriya, A. Kuribayashi and H. Sakiyama, Inorg. Chem., 2002, 41, 4058 CrossRef CAS PubMed.
- H. Yang, J. M. Chen, J. J. Sun, S. P. Yang, J. Yu, H. Tan and W. Li, Dalton Trans., 2009, 2540 RSC.
- S. Zhang, N.-X. Sun, L. Li, Z.-B. Han and Y.-Z. Zheng, RSC Adv., 2014, 4, 5740 RSC.
- (a) Y. Li, W.-Q. Zou, M.-F. Wu, J.-D. Lin, F.-K. Zheng, Z.-F. Liu, S.-H. Wang, G.-C. Guo and J.-S. Huang, CrystEngComm, 2011, 13, 3868 RSC; (b) J. P Zhao, B. W. Hu, Q. Yang, X. F. Zhang, T. L. Hu and X. H. Bu, Dalton Trans., 2010, 56 RSC; (c) Z. Shen, J. L. Zuo, Z. Yu, Y. Zhang, J. F. Bai, C. M. Che, H. K. Fun, J. J. Vittal and X. Z. You, J. Chem. Soc., Dalton Trans., 1999, 3393 RSC; (d) Z.-B. Han, R.-Y. Lu, Y.-F. Liang, Y.-L. Zhou, Q. Chen and M.-H. Zeng, Inorg. Chem., 2012, 51, 674 CrossRef CAS PubMed; (e) M.-Y. Zhang, W.-J. Shan and Z.-B. Han, CrystEngComm, 2012, 14, 1568 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: The TGA curves, simulated, experimental X-ray powder diffraction patterns and X-ray crystallographic file (CIF) for 1–3. CCDC 1013700–1013702. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra10124c |
| This journal is © The Royal Society of Chemistry 2015 |