Fabrication of mesoporous silica nanoparticles hybridised with fluorescent AIE-active quinoline-malononitrile for drug delivery and bioimaging†
Si Yao‡
a,
Andong Shao‡b,
Wenru Zhao*a,
Shaojia Zhuc,
Ping Shic,
Zhiqian Guo*b,
Weihong Zhub and
Jianlin Shi*ad
aLow Dimensional Materials Chemistry Laboratory, School of Materials Science and Engineering, East China University of Science and Technology, Shanghai, 200237, China. E-mail: wenruru@ecust.edu.cn; jlshi@mail.sic.ac.cn
bKey Laboratory for Advanced Materials and Institute of Fine Chemicals, Shanghai Key Laboratory of Functional Materials Chemistry, East China University of Science and Technology, Shanghai 200237, China. E-mail: guozq@ecust.edu.cn
cState Key Laboratory of Bioreactor Engineering, East China University of Science and Technology, 130 Meilong Road, Shanghai 200237, China
dState Key Laboratory of High Performance Ceramics and Superfine Microstructures, Shanghai Institute of Ceramics, Chinese Academy of Sciences, 1295 Dingxi Road, Shanghai, 200050, China
First published on 27th October 2014
Abstract
A novel type of fluorescent mesoporous silica nanoparticle (FMSN) has been successfully synthesised by hybridising mesoporous silica nanospheres with an aggregation-induced emission luminogen, a quinoline-malononitrile derivative. The FMSNs with uniform morphology show excellent AIE-active luminescence and good biocompatibility. Transmission electron microscopy (TEM) demonstrates that the well-ordered mesoporous structure in FMSNs is beneficial for drug delivery. When loaded with doxorubicin (DOX), DOX@FMSNs demonstrate pH-dependent release character and high cytotoxicity towards MCF-7 cancer cells. These FMSNs are expected to be promising multifunctional candidates as both bioimaging agents and drug carriers in cancer therapy.
1 Introduction
In the past few decades, multifunctional mesoporous silica nanoparticles (MSNs) have been tremendously pursued as drug delivery vehicles due to their large surface areas, large pore volumes, tunable pore sizes, good biocompatibilities and ease of surface functionalisation.1–4 In order to endow MSNs with bio-detectability, various functional materials such as organic dyes, semiconductor quantum dots, or magnetic nanoparticles have been incorporated into them.5–13 Among these ingredients, fluorescent MSN-based nanosystems have been intensively investigated14–16 since fluorescence imaging is a powerful and conventional tool in biomedical research. However, such fluorescent MSN-based nanosystems are usually prepared with conventional organic dyes that often suffer from some inherent disadvantages such as the aggregation-caused quenching (ACQ) effect and poor water solubility.17–19Since they were reported by Tang et al. in 2001,20 a class of organic luminogens with an extraordinary aggregation-induced emission (AIE) feature,21–23 which is exactly opposite to the ACQ effect of conventional organic dyes, has received considerable attention. In recent years, the employment of AIE-active materials for biological imaging applications has been extensively explored.24–26 Although the water solubility of AIE-active organic dyes can be improved by modifying the dye molecules with hydrophilic groups,27,28 the sizes and morphologies of the dye aggregates in water are still unstable and difficult to control.29,30 The immobilisation of AIE-active dye molecules onto silica nanoparticles is a good method to improve their solubility in aqueous solution, overcome the issue of fluorescent quenching, and realise morphological control.31–37
Recently, a novel kind of AIE luminogen, quinoline-malononitrile (QM) derivatives, with good stability and relative high-temperature resistance, was reported.27 Attaching the functional groups in the QM building block can be an effective way to realise the modulation of intermolecular aggregating, especially for long wavelength in vivo bio-probes with light-up AIE natures.28,38 In this work, we successfully synthesised fluorescent mesoporous silica nanoparticles (FMSNs) by hybridising MSNs with such novel fluorescent AIE-active QM molecules, making the material luminescent in either water or ethanol. To exploit the potential biomedical applications of FMSNs, the cell imaging and cancer therapy applications of such FMSNs were further investigated.
2 Result and discussion
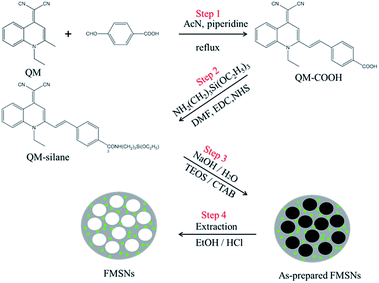
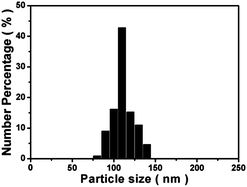
With the goal of fabricating FMSNs with strong light emissions in the solid state, a carboxylated QM derivative (QM-COOH) with AIE attribute was first prepared (Step 1 in Scheme 1).28 The product gives an [M − 1] peak at m/z (366.1240) in its high-resolution mass spectrum (HRMS), as shown in Fig. S1,† confirming the formation of QM-COOH. To covalently hybridise AIE luminogens with silica at the molecular level, the QM-COOH molecules were subsequently silylated (denoted as QM-silane) with 3-aminopropyltriethoxy-silane (APTS) based on the amidation reaction between the carboxyl group of QM-COOH and the amino group of APTS (Step 2). FMSNs were prepared by the hydrolysis and co-condensation of QM-silane and tetraethylorthosilicate (TEOS), self-assembly with cetyltrimethyl ammoniumbromide (CTAB; Step 3), and the subsequent removal of the surfactant CTAB via extraction to form the final AIE dye-incorporated FMSNs (Step 4).The morphology and structure of the FMSNs are shown in the scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images (Fig. 1). The FMSNs are well-formed spheres with uniform particle sizes. The particle size distribution of the FMSNs is narrow and centred at 110 nm (Fig. 2) based on the statistical calculation from a group of TEM images containing over 100 particles, further corroborating their uniform morphology. The TEM image (Fig. 1c) presents a well-ordered mesoporous structure with an average pore diameter of about 3 nm. Fig. 1c also shows that the pore channels open at the particle surface as well as the ordered aligned pores, which is very important for the storage and release of guest molecules such as drugs.
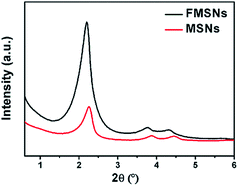
The ordered porous structure of FMSNs and MSNs was further confirmed by X-ray diffraction (XRD). The XRD pattern (Fig. 3) exhibits a high peak in the low-angle region at approximately 2θ = 2° and two small peaks at 2θ = 4° and 2θ = 4.5°, which can be indexed respectively to the (100), (110), and (200) diffractions of the typical hexagonal mesoporous structure.39
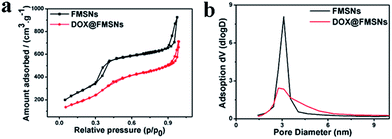
The porosity of FMSNs was evaluated by a nitrogen adsorption–desorption experiment (Fig. 4a); the isotherm exhibits a type IV shape typical of a mesoporous material. The Brunauer–Emmett–Teller (BET) surface area of the FMSNs is 1078 m2 g−1. A narrow pore size distribution of 3.1 nm (Fig. 4b) was also obtained by the Barrett–Joiner–Halenda (BJH) method, which is close to the distribution observed by TEM. The BJH pore volume was estimated to be 1.40 cm3 g−1.
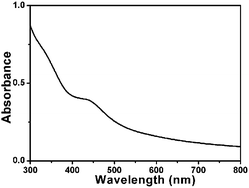
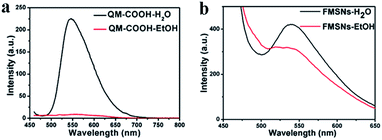
From the UV-vis (Fig. 5) absorbance spectrum of the FMSNs, the characteristic peak of QM-COOH at 450 nm can be obviously observed, indicating that dye molecules were conjugated in the FMSNs. Fig. 6 shows the fluorescence spectra of QM-COOH and FMSNs in water and ethanol. A strong peak at 550 nm appears in the fluorescence spectrum of the aqueous QM-COOH solution (Fig. 6a), while nearly no fluorescence signals were recorded when it was dissolved in ethanol. This is attributed to the fact that the AIE-active QM-COOH molecules aggregate in aqueous solution, while they are highly dispersed in ethanol, similar to the previously reported AIE-active QM derivative dyes.27 When QM-COOH molecules are covalently hybridised in mesoporous silica networks, fluorescence spectra peaks at 550 nm were recorded when the FMSNs were dispersed in either water or ethanol (Fig. 6b). The fluorescence phenomenon of the FMSNs reflects that the AIE dye molecules exist in a certain aggregate state in the mesoporous silica spheres, and the rigid silica networks largely restrict the dissolution of the dye molecules in organic solvent.
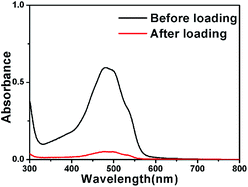
To explore the capability of FMSNs as drug carriers, doxorubicin (DOX), a typical anti-cancer drug, was introduced into the pores of FMSNs. Fig. 7 shows the UV absorbance spectra of 1 mg mL−1 aqueous solutions of DOX before and after interaction with FMSNs. The decrease in the intensity of UV absorbance of DOX after the interaction with FMSNs reflects the reduction of the drug concentration in solution and also the storage of DOX in FMSNs. The DOX loading capacity of FMSNs was determined to be 162 mg g−1 by UV absorbance spectroscopy at 488 nm, the characteristic wavelength of DOX. The storage of DOX was also assessed by N2 adsorption–desorption after DOX loading (Fig. 4); the adsorbed volume of N2 obviously decreases, indicating that DOX molecules were introduced into the mesopores. The same result can also be obtained from the decreases in pore volume (1.1 cm3 g−1) and surface area (765 m2 g−1) after DOX storage.
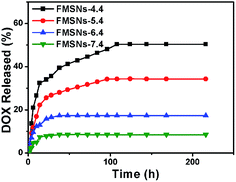
Fig. 8 shows the in vitro DOX release profiles from the DOX-loaded FMSNs (DOX@FMSNs) over a 200 h period in PBS solution at different pH conditions. Obviously, the DOX release rate is pH-dependent. The release was relatively fast during the first 20 h at different pH values. In a pH 7.4 solution, the release reached its maximum (10%) in 15 h. By increasing the acidity of the solution to a pH of 6.4, the release amount reached 17% in 25 h, and in a more acidic solution of pH 5.4, the release amount reached 35% in 90 h. Under strong acidic conditions at pH 4.4, a sustained release followed a fast release within the first 20 h, and over 50% of DOX was released in 100 h. The incomplete release of DOX may be attributed to the electrostatic interactions between DOX and silanol groups in the mesopores. The cationic nature of DOX caused it to interact electrostatically with the negative charges in pores at physiological pH. The zeta potential of FMSNs was about −54.74 mv, much lower than that of MSNs (−17.40 mv); this should be attributed to the incorporation of the negatively charged QM-COOH. As the pH value can be significantly lowered in tumour cells by particle endocytosis compared to the near-neutral conditions outside of tumour cells, this pH-dependent release character is very important and useful.
The intracellular distribution and labelling performance of the FMSNs were demonstrated by incubating DOX@FMSNs with MCF-7 cells for 4 h at 37 °C in the culture medium. The confocal laser scanning microscopy (CSLM) images recorded under the channel of QM-COOH (Fig. 9A1–A3) show that some green fluorescence dots were located in the cell cytoplasm, implying significant internalisation of FMSNs after 4 h of incubation. In addition, the red emission of DOX (Fig. 9B1–B3) can be found not only in almost the same position of the cell cytoplasm, but also in the cell nucleus, suggesting that the taken up DOX molecules were carried by FMSNs via endocytosis, and some were subsequently released into the nucleus.40 When MCF-7 cells were incubated with FMSNs at different concentrations for different times, the intensity of fluorescence increased with increasing nanoparticle concentration, and the fluorescence signal was still detectable in the FMSNs-labelled cells after three generations (Fig. S3 and S4†). CLSM images (Fig. S5†) further confirmed that the endocytosis had little effect on cellular morphology.
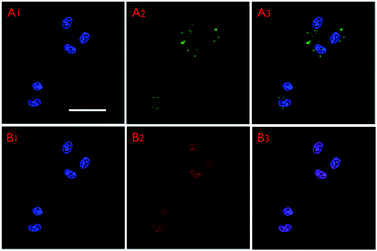 | ||
| Fig. 9 CLSM images of MCF-7 cells incubated with DOX@FMSNs for 4 h. A1–A3: CLSM images of QM-COOH channel; B1–B3: CLSM images of DOX channel. All images share the same scale bar (50 μm). | ||
Flow cytometric analyses were carried out over the MCF-7 cells co-cultured with DOX@FMSNs for 1 h and 4 h. As shown in Fig. 10, the cells incubated for 1 h (b) display over 10 times higher emission intensities than those of the control group (a), and the cells incubated for 4 h (c) exhibit further increases. This quantitatively corroborates the uptake of FMSNs by MCF-7 cells.
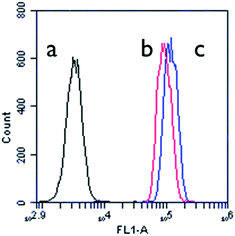 | ||
| Fig. 10 Flow cytometric histogram profiles of MCF-7 cells before (a) and after incubation with DOX@FMSNs for (b) 1 h and (c) 4 h. | ||
To verify whether the released DOX from FMSNs is pharmacologically active, the cytotoxic effect of the DOX@FMSNs on MCF-7 cells was tested. The DOX@FMSNs caused high levels of MCF-7 cell death, similar to free DOX at the same concentration (Fig. 11). This indicated that the DOX delivered by FMSNs retained its pharmaceutical activity. The cytotoxicity against MCF-7 cells demonstrates a dependency on incubation time and concentration. Meanwhile, the FMSNs alone show very low cytotoxicity against 30 MCF-7 cells (Fig. 12) as well as representative normal cells (L02 normal human liver cells).
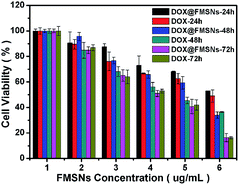 | ||
| Fig. 11 In vitro cell viabilities of MCF-7 cells incubated at same concentration of DOX for 24 h, 48 h and 72 h. | ||
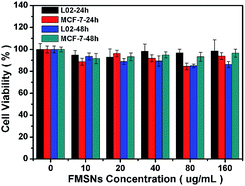 | ||
| Fig. 12 In vitro cell viabilities of L02 cells and MCF-7 cells incubated with FMSNs at different concentrations for 24 h and 48 h. | ||
3 Experimental section
3.1 Synthesis of QM-COOH
QM (150.0 mg, 0.64 mmol) and 4-formylbenzoic acid (105.3 mg, 0.7 mmol) were dissolved in 20 mL of acetonitrile with piperidine (0.5 mL) under argon protection at room temperature. The mixture was then refluxed for 10 h. The solvent was removed under reduced pressure, and the crude product was purified by silica gel chromatography with dichloromethane–methane (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford an orange solid QM-COOH (107.1 mg, 45.6% yield).
1) to afford an orange solid QM-COOH (107.1 mg, 45.6% yield).
3.2 Synthesis of QM-silane
The compound dye molecules QM-COOH were covalently attached to the APTS silane based on an amidation reaction. Briefly, QM-COOH (2 mg) was dissolved in 4 mL DMF. EDC·HCl (20 mg) and NHS (10 mg) as catalyst were introduced to the QM-COOH solution, followed by stirring for 30 min at room temperature. Then APTS (12.7 μL) was added and the solution was stirred magnetically overnight. The QM-silane solution was stored for the later synthesis.3.3 Synthesis of FMSNs
The above QM-silane solution (4 mL) was added to a solution containing H2O (120 mL), NaOH (0.875 mL/2 M) and CTAB (0.25 g). TEOS (1.25 mL) was then added under vigorous stirring. The mixture was allowed to react for 2 h at 80 °C to produce a yellow precipitate. The solid crude product was separated by centrifugation and washed three times with ethanol. Finally, the CTAB surfactant agents were removed by extraction in an acidic ethanol solution (1 mL of HCl (37%) in 100 mL ethanol) at 80 °C for 12 h. This process was repeated twice. The final FMSNs were collected by centrifugation, washing several times with ethanol and distilled water, and drying under vacuum.3.4 DOX storage and release
The resultant FMSNs (50 mg) were suspended and stirred in DOX aqueous solution (1 mg mL−1) for 24 h at room temperature in a dark place. The resulting suspension was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min and dried overnight at 298 K under vacuum. The effective DOX storage capacities of the obtained samples were determined by measuring the difference in the absorption band of DOX (488 nm) in the supernatant before and after loading by UV-vis absorbance spectrometry. DOX-loaded FMSNs (10 mg) were suspended in 4 mL of phosphate buffered saline (PBS) at different pH values (4.4, 5.4, 6.4 and 7.4). The suspensions were placed into dialysis bags with a molecular weight cut-off of 3500 Da and were pre-treated to remove heavy metal and sulphur contaminants. Subsequently, they were placed into beakers containing 36 mL of PBS. The dissolution medium was constantly stirred (∼100 rpm) at 37 °C, and the release process was determined by taking an aliquot of the dissolved fraction at different times. The UV-vis absorbance of the DOX in the dissolved fraction was measured to provide information on the quantity of released DOX.
000 rpm for 20 min and dried overnight at 298 K under vacuum. The effective DOX storage capacities of the obtained samples were determined by measuring the difference in the absorption band of DOX (488 nm) in the supernatant before and after loading by UV-vis absorbance spectrometry. DOX-loaded FMSNs (10 mg) were suspended in 4 mL of phosphate buffered saline (PBS) at different pH values (4.4, 5.4, 6.4 and 7.4). The suspensions were placed into dialysis bags with a molecular weight cut-off of 3500 Da and were pre-treated to remove heavy metal and sulphur contaminants. Subsequently, they were placed into beakers containing 36 mL of PBS. The dissolution medium was constantly stirred (∼100 rpm) at 37 °C, and the release process was determined by taking an aliquot of the dissolved fraction at different times. The UV-vis absorbance of the DOX in the dissolved fraction was measured to provide information on the quantity of released DOX.
3.5 In vitro biocompatibility of FMSNs with L02
Normal human liver cells: For the biocompatibility studies of parent FMSNs with L02 cells, cells were seeded in a 96-well plate at a density of 104 cells per well and cultured in 5% CO2 at 37 °C for 24 h. Parent FMSNs were then added to the media, and the cells were incubated in 5% CO2 at 37 °C for 24 h or 48 h. The concentrations of the parent FMSNs were 0, 10, 20, 40, 80 and 160 μg mL−1. Cell viability was determined by the standard 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay.3.6 In vitro cytotoxicity against MCF-7 cells
For the cytotoxicity analysis of free DOX, parent FMSNs and DOX@FMSNs against MCF-7 cells, cells were seeded in a 96-well plate at a density of 104 cells per well and cultured in 5% CO2 at 37 °C for 24 h. Free DOX was then dispersed in DMSO, and DOX@FMSNs were added to the media. The cells were then incubated in 5% CO2 at 37 °C for 24 h, 48 h or 72 h and in parent FMSNs for 24 h or 48 h. The concentrations of DOX were 0, 0.5, 1.0, 2.5, 10 and 20 μg mL−1, and the concentrations of parent FMSNs were 0, 10, 20, 40, 80 and 160 μg mL−1. Cell viability was determined by MTT assay. The statistical analysis of the experimental data utilised the Student's t-test. A p-value of less than 0.05 was considered statistically significant. Each data point is represented as mean ± standard deviation (SD) of eight independent experiments (n = 8, n indicates the number of wells in a plate for each experimental condition).3.7 Flow cytometric analyses
MCF-7 cells were seeded into a six-well plate at a density of 104 cells per well, and MCF-7 cells were then incubated at 37 °C in 5% CO2 for 24 h. The medium was removed, and the cells were washed with PBS buffer solution (pH = 7.4). The cells were then incubated with DOX@FMSNs (10 μg mL−1 DOX) for 1 h or 4 h at 37 °C.3.8 Cell imaging
MCF-7 cells were grown overnight on a plasma-treated 25 mm round cover slip mounted onto a 35 mm Petri dish with an observation window. The living cells were stained with free DOX, FMSNs and DOX@FMSNs for 4 h. The cells were washed by PBS and dyed with 10 μg mL−1 of DAPI for 5 min followed by washing with PBS. Finally, the samples were excited at 330–380 nm for nuclei and 450 nm for fluorescent molecules in FMSNs and 488 nm for DOX. The cells were imaged under CLSM.4 Conclusion
In summary, uniform FMSNs with particle sizes of around 110 nm were successfully prepared by hybridising MSNs with an AIE-active luminogen, a QM derivative. The luminescence in either water or ethanol endowed the FMSNs with great potential for fluorescent bioimaging applications. Since DOX, a typical anticancer drug, can be effectively stored in the pores of FMSNs and released pH-dependently, the DOX-loaded FMSNs demonstrated high cytotoxicity for MCF-7 cancer cells. In contrast, the FMSNs showed no effect on the viability of normal human liver cells (L02). Therefore, the novel FMSNs are expected to be promising multifunctional candidates for both bioimaging and cancer therapy.Acknowledgements
This work was supported by 973 Program (Grant no. 2012CB933602), the National Natural Science Foundation of China (Grant no. 51372084), the Natural Science Foundation of Shanghai (Grant no. 14ZR1410100), the Fundamental Research Funds for the Central Universities (222201313010), and the Nano-Special Foundation for Shanghai Committee of Science and Technology (12nm0502600).Notes and references
- Y. Cui, H. Dong, X. Cai, D. Wang and Y. Li, ACS Appl. Mater. Interfaces, 2012, 4, 3177–3183 CAS.
- Q. Zhang, F. Liu, K. T. Nguyen, X. Ma, X. Wang, B. Xing and Y. Zhao, Adv. Funct. Mater., 2012, 22, 5144–5156 CrossRef CAS.
- S. H. Wu, C. Y. Mou and H. P. Lin, Chem. Soc. Rev., 2013, 42, 3862–3875 RSC.
- T. Suteewong, H. Sai, R. Hovden, D. Muller, M. S. Bradbury, S. M. Gruner and U. Wiesner, Science, 2013, 340, 337–341 CrossRef CAS PubMed.
- J. Yang, Y. Deng, Q. Wu, J. Zhou, H. Bao, Q. Li, F. Zhang, F. Li, B. Tu and D. Zhao, Langmuir, 2010, 26, 8850–8856 CrossRef CAS PubMed.
- Y. Shan, J. J. Xu and H. Y. Chen, Nanoscale, 2011, 3, 2916–2923 RSC.
- H. Wu, G. Liu, S. Zhang, J. Shi, L. Zhang, Y. Chen, F. Chen and H. Chen, J. Mater. Chem., 2011, 21, 3037–3045 RSC.
- W. Zhao, J. Gu, L. Zhang, H. Chen and J. Shi, J. Am. Chem. Soc., 2005, 127, 8916–8917 CrossRef CAS PubMed.
- W. Zhao, H. Chen, Y. Li, L. Li, M. Lang and J. Shi, Adv. Funct. Mater., 2008, 18, 2780–2788 CrossRef CAS.
- Y. Ma, Y. Liu, X. Zhou and C. Liu, Micro Nano Lett., 2013, 8, 302–304 CAS.
- Z. Lin, X. Fei, Q. Ma, X. Gao and X. Su, New J. Chem., 2014, 38, 90–96 RSC.
- Y. Shan, J. J. Xu and H. Y. Chen, Nanoscale, 2011, 3, 2916–2923 RSC.
- R. Gui, Y. Wang and J. Sun, Colloids Surf., B, 2014, 113, 1–9 CrossRef CAS PubMed.
- C. H. Lee, S. H. Cheng, Y. J. Wang, Y. C. Chen, N. T. Chen, J. Souris, C. T. Chen, C. Y. Mou, C. S. Yang and L. W. Lo, Adv. Funct. Mater., 2009, 19, 215–222 CrossRef CAS.
- M. Xie, H. Shi, K. Ma, H. Shen, B. Li, S. Shen, X. Wang and Y. Jin, J. Colloid Interface Sci., 2013, 395, 306–314 CrossRef CAS PubMed.
- B. Ahn, J. Park, K. Singha, H. Park and W. J. Kim, J. Mater. Chem. B, 2013, 1, 2829–2836 RSC.
- C. Huang, S. Barlow and S. R. Marder, J. Org. Chem., 2011, 76, 2386–2407 CrossRef CAS PubMed.
- A. C. Grimsdale, K. L. Chan, R. E. Martin, P. G. Jokisz and A. B. Holmes, Chem. Rev., 2009, 109, 897–1091 CrossRef CAS PubMed.
- W. Qin, D. Ding, J. Liu, W. Z. Yuan, Y. Hu, B. Liu and B. Z. Tang, Adv. Funct. Mater, 2012, 22, 771–779 CrossRef CAS.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740–1741 RSC.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC.
- H. Li, X. Zhang, Z. Chi, B. Xu, W. Zhou, S. Liu, Y. Zhang and J. Xu, Org. Lett., 2011, 13, 556–559 CrossRef CAS PubMed.
- Z. Zhao, S. Chen, X. Shen, F. Mahtab, Y. Yu, P. Lu, J. W. Y. Lam, H. S. Kwok and B. Z. Tang, Chem. Commun., 2010, 46, 686–688 RSC.
- W. Qin, D. Ding, J. Liu, W. Z. Yuan, Y. Hu, B. Liu and B. Z. Tang, Adv. Funct. Mater., 2012, 22, 771–779 CrossRef CAS.
- Z. Zhao, J. Geng, Z. Chang, S. Chen, C. Deng, T. Jiang, W. Qin, J. W. Y. Lam, H. S. Kwok and H. Qiu, J. Mater. Chem., 2012, 22, 11018–11021 RSC.
- J. Geng, K. Li, D. Ding, X. Zhang, W. Qin, J. Liu, B. Z. Tang and B. Liu, Small, 2012, 8, 3655–3663 CrossRef CAS PubMed.
- C. Shi, Z. Guo, Y. Yan, S. Zhu, Y. Xie, Y. S. Zhao, W. Zhu and H. Tian, ACS Appl. Mater. Interfaces, 2013, 5, 192–198 CAS.
- A. Shao, Z. Guo, S. J. Zhu, S. Zhu, P. Shi, H. Tian and W. Zhu, Chem. Sci., 2014, 5, 1383–1389 RSC.
- X. Zhang, X. Zhang, S. Wang, M. Liu, Y. Zhang, L. Tao and Y. Wei, ACS Appl. Mater. Interfaces, 2013, 5, 1943–1947 CAS.
- X. Zhang, X. Zhang, S. Wang, M. Liu, L. Tao and Y. Wei, Nanoscale, 2013, 5, 147–150 RSC.
- K. Hayashi, M. Nakamura, H. Miki, S. J. Ozaki, M. Abe, T. Matsumoto and K. Ishimura, Adv. Funct. Mater., 2012, 22, 3539–3546 CrossRef CAS.
- M. T. Hurley, Z. Wang, A. Mahle, R. Daniel, Q. Liu, D. S. English, M. R. Zachariah, D. Stein and P. D. Shong, Adv. Funct. Mater., 2013, 23, 3335–3343 CrossRef CAS.
- Y. Yu, C. Feng, Y. Hong, J. Liu, S. Chen, K. M. Ng, K. Q. Luo and B. Z. Tang, Adv. Mater., 2011, 23, 3298–3302 CrossRef CAS PubMed.
- M. Faisal, Y. Hong, J. Liu, Y. Yu, J. W. Y. Lam, A. Qin, P. Lu and B. Z. Tang, Chem.–Eur. J, 2010, 16, 4266–4272 CrossRef CAS PubMed.
- F. Mahtab, Y. Yu, J. W. Y. Lam, J. Liu, B. Zhang, P. Lu, X. Zhang and B. Z. Tang, Adv. Funct. Mater., 2011, 21, 1733–1740 CrossRef CAS.
- F. Mahtab, J. W. Y. Lam, Y. Yu, J. Liu, W. Yuan, P. Lu and B. Z. Tang, small, 2011, 10, 1448–1455 CrossRef PubMed.
- M. Li, J. W. Y. Lam, F. Mahtab, S. Chen, W. Zhang, Y. Hong, J. Xiong, Q. Zheng and B. Z. Tang, J. Mater. Chem. B, 2013, 1, 676–684 RSC.
- D. Lu, J. Lei, L. Wang and J. Zhang, J. Am. Chem. Soc., 2012, 134, 8746–8749 CrossRef CAS PubMed.
- J. Gu, S. Su, M. Zhu, Y. Li, W. Zhao, Y. Duan and J. Shi, Microporous Mesoporous Mater., 2012, 161, 160–167 CrossRef CAS PubMed.
- K. Cohen, R. Emmanuel, E. K. Finfer, D. Shabat and D. Peer, ACS Nano, 2014, 8, 2183–2195 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Characterization of QM-COOH See DOI: 10.1039/c4ra10114f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |