A highly selective turn-on fluorescent probe based on semi-cyanine for the detection of nitroreductase and hypoxic tumor cell imaging†
Jun Yuan‡
a,
Yu-Qiong Xu‡a,
Nan-Nan Zhoub,
Rui Wangb,
Xu-Hong Qiana and
Yu-Fang Xu*a
aShanghai Key Laboratory of Chemical Biology, State Key Laboratory of Bioreactor Engineering, School of Pharmacy, East China University of Science and Technology, Shanghai 200237, China. E-mail: yfxu@ecust.edu.cn
bShanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, Shanghai 200237, China
First published on 24th October 2014
Abstract
Based on a semi-cyanine fluorophore, a selective turn-on fluorescent probe semi-CyHP for the detection of nitroreductase (NTR) and hypoxia was designed and synthesized. It can be activated by NTR and to restore the pull–push electronic systems of semi-CyHF, which strongly fluoresces at 556 nm. Besides, the investigation of the hypoxic tumor cell imaging with semi-CyHP was significant with minimal endogenous interference.
Nitroreductases are a family of evolutionarily related proteins, which participate in the reduction of those compounds containing the nitro functional group by using the flavin mononucleotide as a cofactor.1,2 NTR has been used not only to activate nitrofuran antibiotics but also to eliminate pervasive nitroaromatic pollutants.3–6 Moreover, decrease of oxygen levels in tumor cells is often accompanied by the apparent increase of endogenous nitroreductase activity.7,8 However, hypoxia is a common feature of solid tumors and renders tumor cells higher resistance towards therapy by preventing the proper metabolism of various anticancer drugs.9,10 Hypoxia results in reductive stress and over expression of nitroreductase (NTR), azoreductase and quinone reductase.11,12 And it is believed that the technique of tumor targeting is of significance for the diagnosis and treatment of cancer.13–15 Therefore, the detection of hypoxia and NTR has been a feasible tool for the diagnosis of tumor cells and warrants robust detection for biological and environmental studies.
Various approaches for detection of NTR and hypoxia are available, among which fluorescence probe has attracted much attention because of its great temporal and spatial sampling capability as well as high sensitivity.7,8,16–19 Nitroaromatics are readily metabolized by NTR in a stepwise reduction pathway by cellular nitroreductase under hypoxic condition.20 Probe with pimonidazole as a hypoxia marker for nitroreductase was reported by Nagasawa et al.21 And based on that azo group has excellent response to hypoxia, the first near-infrared fluorescent probe was developed by Nagano et al.22
Cyanines are a facile family of fluorescent dyes. Depending on the length of polymethine backbone, the entire spectrum from IR to UV may be covered. Cyanines have been extensively used in biomedical imaging. There are few developments in the structural modifications of cyanines, which achieves the diversity and novelty of chemical probes. Because of the unique nature of indole group, the main component of cyanines, it is usually covalently linked to other existed fluorophores,23,24 which increases the conjugated system and results in the improvement of its spectroscopic properties. Doron et al. developed a new paradigm for generation of novel class of turn-on NIR cyanine-based probes.25,26 A distinctive change of π-electronic system leads to generation of a cyanine dye with strong NIR fluorescence.27 Although previous cyanine-based fluorescent probes for nitroreductase and hypoxia are of satisfactory by using nitroimidazole or p-nitrobenzyl as the receptor, the receptor is not involved in the composition of the fluorophore.16,18 Herein, we describe a novel cyanine-based turn-on fluorescent probe (semi-CyHP) for detection of NTR and hypoxia imaging and the corresponding reduction product semi-CyHF was achieved by organic synthesis for the spectroscopic studies in solution.
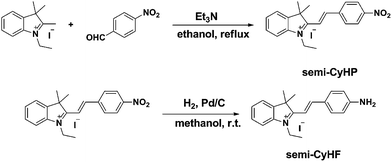
The p-nitrophenyl group can be conveniently converted into p-aminophenyl by nitroreductase or under hypoxic condition. Therefore, our strategy for the construction of this hypoxia probe semi-CyHP relies on tethering the p-nitrophenyl group to the indole moiety via an alkenyl linkage (Scheme 1). The p-nitrophenyl group is not only a receptor for NTR but also an excellent fluorescence-quench group.28 The electron-withdrawing nitro group inhibits the ICT effect and results in a weak fluorescence emission. Upon reduction, the amino group of semi-CyHF will reconstruct the electronic push–pull system and the fluorescence emission will be restored (Fig. 1).
The probe semi-CyHP was readily synthesized by condensing p-nitrobenzaldehyde and 1,2,3-trimethyl-3-methyl-benzo indole iodized salt. The bioreduction product semi-CyHF was also synthesized by reduction of semi-CyHP (Scheme 1).
Next, we studied their spectral properties in chemical, enzymatic and cell media. Spectroscopic evaluation of semi-CyHP and semi-CyHF was carried out under physiological conditions at 37 °C in PBS buffer (pH = 7.0, 0.01 M) with 1% DMSO as co-solvent (Fig. S1†). The probe semi-CyHP shows a maximal absorption at 385 nm. The reductive semi-CyHF has a strong absorption peak at 490 nm and a strong fluorescence peak at 556 nm. When semi-CyHP was incubated with the NTR, a drastic enhancement of fluorescence intensity at 556 nm was observed (Fig. S2†).
Then, the assay of the probe semi-CyHP toward the reduction of NTR was performed as described previously.7,19 The fluorescence of the solution of probe semi-CyHP (10 μM) was undetectable when excited at around 490 nm. After that 17.5 μg mL−1 of NTR was added, an apparent fluorescence enhancement at around 556 nm was observed (Fig. 2). It can be drawn that the reduction of the probe semi-CyHP was underway and the reductive product semi-CyHF was formed as expected. It was estimated that ca. 95% of semi-CyHP was converted to semi-CyHF (Fig. S3†). The drastic color change concomitant to reduction of semi-CyHP was easily noticed to naked eyes. It is also found that the fluorescence enhancement and maximal fluorescence intensity (around 556 nm) are connected to the initial concentrations of semi-CyHP (1–20 μM) (Fig. S4†).
Additionally, the fluorescence enhancement and maximal fluorescence intensity of the semi-CyHP (10 μM) varied according to the exposed doses of NTR. A linear relationship between the probe and NTR was established at 2.5–17.5 μg mL−1 and the detection limit was deduced to be 40 ng mL−1 within 30 min (Fig. 3). Because of its sensitivity for detection of NTR, it implied the potential for application in biological systems.
For further application in biological system, the selectivity of semi-CyHP towards other biological reducing agents was under investigation. Thiols have been suggested to be the electron providers for reductive activation of various hypoxia and prodrug.29–31 Therefore, the probe semi-CyHP was treated with biorelevant thiols, such as homocysteine (Hcy), glutathione (GSH), cysteine (Cys), dithiothreitol (DTT) and β-nicotinamide adenine dinucleotide (NADH). Under normal physiological condition, the concentration of reduced biological reductants is far lower than 1 mM. As shown in Fig. 4, incubation with biological thiols (2 mM) did not induce any noticeable signal modulation. And the selectivity was also tested in the mixture of NTR with these thiols, the fluorescence intensity of semi-CyHF increased slightly, even after reaction for 90 min under the same condition. Also, the impact of NADH or NTR on the probe was undergone respectively (Fig. S5†). The remarkable fluorescence emission with 85-fold enhancement at around 556 nm was observed by incubation of semi-CyHP with NADH (50 equiv.) and NTR.
These results suggested that those thiols employed in the experiments exhibited no interferences and our probe semi-CyHP has potentials for selectively monitoring NTR under physiological conditions.
The MTT assay showed that semi-CyHP exhibited little cytotoxicity to A549 cell at 0–10 μM (Fig. S6†). A549 cells were incubated with F12 culture medium containing 10% FBS under hypoxic and normal conditions, respectively, at 37 °C for 12 h and then washed 3 times with PBS (pH 7.0) and treated with 5 μM semi-CyHP in FBS-free F12 culture medium for 1 h. The changes of fluorescence intensity were measured using an inverted fluorescence microscope (Fig. 5).
It was obvious that A549 cells treated with the probe semi-CyHP under normoxic conditions showed nearly no fluorescence enhancement (Fig. 5a). Conversely, lots of drastic fluorescence spots appeared within the cells with the same reagents incubated under hypoxic conditions (Fig. 5c). These results clearly demonstrated that semi-CyHP is capable of in vitro imaging of hypoxia in solid tumors.
In summary, we have developed a novel selective and sensitive fluorescent probe semi-CyHP for the detection of NTR and hypoxia. The probe was activated by reaction with NTR and NADH under physiological conditions, to form the reduction product semi-CyHF, leading to a 85 fold fluorescence emission enhancement at ca. 556 nm. And its potentials for imaging applications were exhibited with hypoxic A549 cells.
Acknowledgements
We are grateful for the financial support from the State Key Program of National Natural Science of China (21236002), the National Basic Research Program of China (2010CB126100), the National High Technology Research and Development Program of China (2011AA10A207). We appreciate Professor Youjun Yang (School of Pharmacy, East China University of Science and Technology) for the improvements in this paper.Notes and references
- I. M. de Oliveira, J. A. P. Henriques and D. Bonatto, Biochem. Biophys. Res. Commun., 2007, 355, 919–925 CrossRef PubMed.
- H. J. Hecht, H. Erdmann, H. J. Park, M. Sprinzl and R. D. Schmid, Nat. Struct. Biol., 1995, 2, 1109–1114 CrossRef CAS PubMed.
- J. C. Spain, Annu. Rev. Microbiol., 1995, 49, 523–555 CrossRef CAS PubMed.
- P. R. Race, A. L. Lovering, R. M. Green, A. Ossor, S. A. White, P. F. Searle, C. J. Wrighton and E. I. Hyde, J. Biol. Chem., 2005, 280, 13256–13264 CrossRef CAS PubMed.
- A. Caballero, J. J. Lázaro, J. L. Ramos and A. Esteve-Núñez, Environ. Microbiol., 2005, 7, 1211–1219 CrossRef CAS PubMed.
- A. Çelik and G. Yetiş, Bioorg. Med. Chem., 2012, 20, 3540–3550 CrossRef PubMed.
- L. Cui, Y. Zhong, W. Zhu, Y. Xu, Q. Du, X. Wang, X. Qian and Y. Xiao, Org. Lett., 2011, 13, 928–931 CrossRef CAS PubMed.
- Z. Li, X. Li, X. Gao, Y. Zhang, W. Shi and H. Ma, Anal. Chem., 2013, 85, 3926–3932 CrossRef CAS PubMed.
- K. J. Williams, M. R. Albertella, B. Fitzpatrick, P. M. Loadman, S. D. Shnyder, E. C. Chinje, B. A. Telfer, C. R. Dunk, P. A. Harris and I. J. Stratford, Mol. Cancer Ther., 2009, 8, 3266–3275 CrossRef CAS PubMed.
- E. T. Shinohara and A. Maity, Curr. Mol. Med., 2009, 9, 1034–1045 CrossRef CAS.
- J. M. Brown and W. R. Wilson, Nat. Rev. Cancer, 2004, 4, 437–447 CrossRef CAS PubMed.
- Y. Chen and L. Hu, Med. Res. Rev., 2009, 29, 29–64 CrossRef CAS PubMed.
- Z. Yu, R. M. Schmaltz, T. C. Bozeman, R. Paul, M. J. Rishel, K. S. Tsosie and S. M. Hecht, J. Am. Chem. Soc., 2013, 135, 2883–2886 CrossRef CAS PubMed.
- C. Bhattacharya, Z. Yu, M. J. Rishel and S. M. Hecht, Biochemistry, 2014, 53, 3264–3266 CrossRef CAS PubMed.
- B. R. Schroeder, M. I. Ghare, C. Bhattacharya, R. Paul, Z. Yu, P. A. Zaleski, T. C. Bozeman, M. J. Rishel and S. M. Hecht, J. Am. Chem. Soc., 2014, 136, 13641–13656 CrossRef CAS PubMed.
- Y. Shi, S. Zhang and X. Zhang, Analyst, 2013, 138, 1952–1955 RSC.
- Z. Li, X. Gao, W. Shi, X. Li and H. Ma, Chem. Commun., 2013, 49, 5859–5861 RSC.
- K. Xu, F. Wang, X. Pan, R. Liu, J. Ma, F. Kong and B. Tang, Chem. Commun., 2013, 49, 2554–2556 RSC.
- T. Guo, L. Cui, J. Shen, W. Zhu, Y. Xu and X. Qian, Chem. Commun., 2013, 49, 10820–10822 RSC.
- C. Hernández, E. Santamatilde, K. J. McCreath, A. M. Cervera, I. Díez, D. Ortiz-Masiá, N. Martínez, S. Calatayud, J. V. Esplugues and M. D. Barrachina, Br. J. Pharmacol., 2009, 156, 262–272 CrossRef PubMed.
- K. Okuda, Y. Okabe, T. Kadonosono, T. Ueno, B. G. M. Youssif, S. Kizaka-Kondoh and H. Nagasawa, Bioconjugate Chem., 2012, 23, 324–329 CrossRef CAS PubMed.
- K. Kiyose, K. Hanaoka, D. Oushiki, T. Nakamura, M. Kajimura, M. Suematsu, H. Nishimatsu, T. Yamane, T. Terai, Y. Hirata and T. Nagano, J. Am. Chem. Soc., 2010, 132, 15846–15848 CrossRef CAS PubMed.
- L. Li, Z. Li, W. Shi, X. Li and H. Ma, Anal. Chem., 2014, 86, 6115–6120 CrossRef CAS PubMed.
- J.-A. Richard, M. Massonneau, P.-Y. Renard and A. Romieu, Org. Lett., 2008, 10, 4175–4178 CrossRef CAS PubMed.
- N. Karton-Lifshin, E. Segal, L. Omer, M. Portnoy, R. Satchi-Fainaro and D. Shabat, J. Am. Chem. Soc., 2011, 133, 10960–10965 CrossRef CAS PubMed.
- N. Karton-Lifshin, L. Albertazzi, M. Bendikov, P. S. Baran and D. Shabat, J. Am. Chem. Soc., 2012, 134, 20412–20420 CrossRef CAS PubMed.
- E. Kisin-Finfer and D. Shabat, Bioorg. Med. Chem., 2013, 21, 3602–3608 CrossRef CAS PubMed.
- K. Mutai, Bull. Chem. Soc. Jpn., 1971, 44, 2537–2541 CrossRef CAS.
- M. M. Paz, Chem. Res. Toxicol., 2009, 22, 1663–1668 CrossRef CAS PubMed.
- S. E. Wolkenberg and D. L. Boger, Chem. Rev., 2002, 102, 2477–2496 CrossRef CAS PubMed.
- M. M. Paz and M. Tomasz, Org. Lett., 2001, 3, 2789–2792 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental section, NMR and MS copies of new compounds and Fig. S1. See DOI: 10.1039/c4ra10044a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2014 |