Solvothermal synthesis, crystal structure and photoluminescence properties of four Cd(ii) coordination polymers with different topological structures†
Abstract
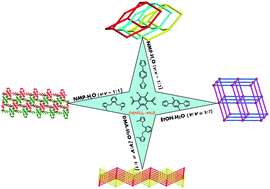
Solvothermal reactions of tetrabromoterephthalic (H2tbtpa) acid and different bis-imidazoles with cadmium nitrate provided four new Cd(II) coordination polymers (CPs), namely, {[Cd(bip)(tbtpa)]·H2O}n (1), {[Cd2(bibp)(tbtpa)2(H2O)4]·H2O}n (2), {[Cd(1,2-mbix)(tbtpa)]·H2O}n (3) [Cd(1,2-mbix)(tbtpa)]n (4), (bip = 1,4-di(1H-imidazol-1-yl)benzene, bibp = 1,1′-(2,5-dimethyl-1,4-phenylene)bis(1H-imidazole), 1,2-mbix = 1,2-bis((2-methyl-1H-imidazol-1-yl)methyl)benzene, H2tbtpa = tetrabromoterephthalic acid). All of the compounds have been structurally characterized by single-crystal X-ray diffraction analyses and further characterized by elemental analyses, IR spectroscopy, powder X-ray diffraction (PXRD), and thermogravimetric analyses (TGA). Single crystal X-ray diffraction analysis reveals that compound 1 exhibits a 3D diamond-type (dia, 66) framework of 3-fold interpenetration. Compound 2 displays a noninterpenetrated 3D network with the classical pcu topology. Compound 3 exhibits a polyrotaxane-like 2D + 2D → 2D layer with 44-sql topology and the 2D sheets further formed a 3D framework by π⋯π interaction. Compound 4 is a 2D 44-sql network and the adjacent 2D networks are packed parallel in a ⋯ABAB⋯fashion. Moreover, the thermal stability and photoluminescence properties of the compounds were investigated.

 Please wait while we load your content...
Please wait while we load your content...