A facile and efficient preparation of anatase titania nanoparticles in micelle nanoreactors: morphology, structure, and their high photocatalytic activity under UV light illumination†
Abstract
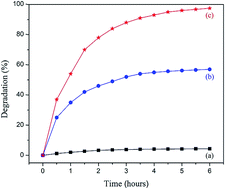
In the present study, synthesis of titania (titanium dioxide, TiO2) nanoparticles, which show light absorption characteristics in the ultraviolet region, was successfully achieved in the CTAB micelle nanoreactors at room temperature. The shape (or surface morphology), size, elemental composition, crystal structure, and optical properties of these particles were characterized by scanning electron microscope (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), and ultraviolet-visible absorption (UV-Vis) spectroscopy. Besides, our proposed method allowed for the successful preparation of highly crystalline and well-dispersed anatase titania nanoparticles with a phase-pure. Evaluation of titania nanoparticles' photocatalytic activity was performed through degrading the methylene blue (MB) dye under ultraviolet light irradiation. Compared to the commercial P25 TiO2 powder, this result is indicative of a high photocatalytic MB dye degradation activity. Also, the effect of the amount of TiO2 nanoparticles in the MB dye degradation has been investigated in detail. Finally, the stability of photocatalytic activity of those nanoparticles has been evaluated.

 Please wait while we load your content...
Please wait while we load your content...