Tungstate adsorption onto oxisols in the vicinity of the world's largest and longest-operating tungsten mine in China
Abstract
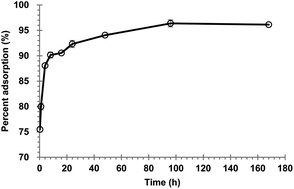
Tungstate adsorption in soils is critical to understand tungstate mobility and bioavailability, but study of this is lacking. The objectives of this study are to investigate the kinetics and isotherms of tungstate adsorption onto oxisol samples in the vicinity of the world's largest and longest-operating tungsten mine in China. In addition, the effects of pH, ionic strength, and phosphate anion on tungstate adsorption onto the soil were studied. Results show that the tungstate adsorption kinetics is fitted best by a pseudo-second order model. Micropore (intraparticle) diffusion and ultramicropore (within clays) diffusion are generally the adsorption-limiting mechanisms. Tungstate adsorption isotherms are fitted well by both Langmuir and Freundlich models. The maximal adsorption capacity of the oxisol sample is 10.67 mmol kg−1, while the distribution coefficient is 12.60 (mmol kg−1) (mol L−1)−1/n. Tungstate adsorption decreased from 96.1% to 90.2% with the pH increase from 4.93 to 5.23, while it increased from 90.1% to 95.5% with the increase of ionic strength from 0.01 M to 0.1 M NaCl. With the increase of phosphate concentration from 0.008 mM to 0.215 mM, tungstate adsorption slightly decreased from 2.32 mmol kg−1 to 1.97 mmol kg−1. These results demonstrate that tungstate might be adsorbed onto the tungstate-specific adsorption sites of the soil minerals mainly via inner-sphere complexation. Despite the soil containing a high tungsten content (e.g. 21.9 mg kg−1), it still has a high tungstate adsorption capacity.

 Please wait while we load your content...
Please wait while we load your content...