Simple and effective route for synthesis of parvaquone, an antiprotozoal drug†
Pravin C. Patil‡
and
Krishnacharya G. Akamanchi*
Department of Pharmaceutical Sciences and Technology, Institute of Chemical Technology, Matunga, Mumbai-400 019, India. E-mail: kgap@rediffmail.com; Fax: +91-22-33611020; Tel: +91-22-33612214
First published on 22nd October 2014
Abstract
Parvaquone, an antiprotozoal agent against Theileria parva, was synthesized in 33.8% overall yield by using cheap, commercially available raw materials. A key intermediate, 2-cyclohexyl-1-naphthol, was synthesized in 86% yield by cyclohexylation of 1-naphthol and further converted into parvaquone in good yield through reaction sequences such as oxidation and epoxidation followed by isomerization.
Naphthoquinones and substituted naphthoquinones articulate a broad spectrum of biological activities such as antibacterial, antifungal, anti-inflammatory, antithrombotic, antiplatelet, antibiotic, antiallergic, and apoptosis.1 Naphthoquinones substituted with an alicyclic ring at the 2 or 3 position are considered highly effective agents for suppression of trophozoites in ducks, chickens, canaries and monkeys infected with mosquito- or blood-induced malaria. These compounds are capable of destroying fully developed exoerythrocytic forms of malaria parasites.2
Theileriosis (East Coast fever), a cattle disease, is caused by the microscopic parasite Theileria parva and mainly found in central and eastern Africa. Theileriosis is among the most destructive livestock diseases in Africa, causing an annual loss of 1.1 million cattle and more than 250 million are considered at risk.3 The disease had a high mortality rate up to the 1970s due to the lack of effective treatment; mortality can be 100%, with death occurring within a month after the initial attachment of infected ticks.4 2-cyclohexyl-1,4-naphthoquinone (parvaquone, 1) and 2-((4-tert-butylcyclohexyl)methyl)-3-hydroxy-1,4-naphthoquinone (buparvaquone) have been effective in treating theileriosis since their discovery.3,4 Parvaquone, marketed as Clexon, is an analogue for menoctone, which had been identified as having anti-theilerial activity against Theileria parva.5 Unfortunately, structural complexity and high manufacturing costs prohibited further development of menoctone. Recently, resistance of Theileria parva to buparvaquone has been reported.6 By considering these limitations related to menoctone and buparvaquone, the importance of parvaquone is significantly changing.
The first method for free radical-mediated alkylation of naphthoquinone was developed by Fieser et al. in 1946 by generating free radicals from corresponding diacyl peroxide in the presence of 2-hydroxy-1,4-naphthoquinone (lawsone) to give corresponding alkylated naphthoquinones.7 The method provided inferior yield (5–6%) of alkylated naphthoquinones and generated numerous side products during the course of reaction. However, these protocols were not applied toward synthesis of parvaquone 1.
In 1951, Fieser et al. further developed a method for synthesis of 1 by generating cyclohexyl radicals from cyclohexanecarboperoxoic acid A in the presence of lawsone provides 1 in 45% yield2 (Scheme 1, method a). In 1968, Amini et al. modified Fieser's general method for alkylation of naphthoquinone by replacing corresponding diacyl peroxide with tert-butyl peroxide in respective hydrocarbons and the developed protocol was extended toward synthesis of 1 by using cyclohexane B in combination with tert-butyl peroxide in presence of lawsone8 (Scheme 1, method b). Khambay et al. demonstrated a new method for synthesis of 1 through generating cyclohexyl radicals from cyclohexane carboxylic acid C using silver nitrate/ammonium persulfate in the presence of lawsone and afforded 1 in 25% yield9 (Scheme 1, method c).
 | ||
| Scheme 1 Different routes for cyclohexyl radical mediated synthesis of parvaquone starting from lawsone. | ||
In addition to cyclohexyl radical-mediated transformations, a new approach for the synthesis of 1 has been discussed by Leach and Urquhart et al. by condensing 1,4-isochromandione 7 with cyclohexanecarbaldehyde 8 followed by rearrangement of the resulting aldol product using sodium methoxide in methanol to afford an overall 77.3% yield of 1 (ref. 10) (Scheme 2).
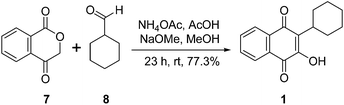 | ||
| Scheme 2 Synthesis of parvaquone through condensation of 1,4-isochromandione with cyclohexanecarbaldehyde. | ||
Most of these single-step transformations for the synthesis of 1 have shortcomings such as the need for specialized starting materials, use of hazardous peroxides in the presence of oxidants, harsh reaction conditions, costly raw materials, and low yields due to byproduct formation, especially in free radical-mediated routes.2,8,9
To overcome aforementioned problems, multistep synthesis through the ring construction approach has been recently established by Pena-Cabrera et al. for the synthesis of 1 (ref. 11a) and its derivatives11b starting from diisopropyl squarate. Use of Grignard reagents, cryogenic reaction conditions, use of trifluoroacetic anhydride and boron tribromide under an inert atmosphere limited the manufacturing viability of this process. Considering these inadequacies, the development of an adaptable method for the synthesis of 1 using cheap raw materials and mild reaction conditions in an open atmosphere still remains a significant challenge.
Herein we report a simple and effective synthesis of parvaquone developed from cheap and commercially available raw materials. A new reaction system has been established for the synthesis of a key intermediate, 2-cyclohexyl-1-naphthol 4, by cyclohexylation of 1-naphthol 2 using cyclohexanol 3 in the presence of p-toluenesulfonic acid. The obtained 4 was oxidized by using a mild oxidant such as 30% hydrogen peroxide in the presence of hydrochloric acid to afford 2-cyclohexyl-1,4-naphthoquinone 5. The resulting compound 5 was further converted into parvaquone, 1, by epoxidation followed by isomerization. The general synthetic route is depicted in Scheme 3.
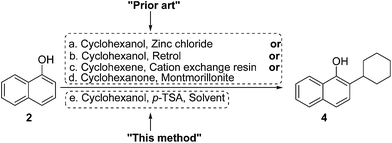
During the literature survey related to the synthesis of parvaquone, we determined that the most significant challenge was introduction of the cyclohexyl moiety, and therefore an alternative key intermediate, 4, was selected toward simplification of cyclohexylation process. Cyclohexylation of naphthol has been reported via catalytic methods using catalysts such as zinc chloride12a (Scheme 4, path a), Retrol (an acid-activated bleaching earth,12b Scheme 4, path b), cation exchange resin (Scheme 4, path c),12c and montmorillonite12d (Scheme 4, path d) in the presence of various cyclohexylating counterparts such as cyclohexanol, cyclohexene and cyclohexanone. Most of these reported methods have disadvantages of affording very low yields along with necessary high temperature.
With this background, we attempted an alternative approach for synthesis of 4. Based on preliminary observations from trial experiments, cyclohexanol was chosen as the cyclohexylating agent over cyclohexene and cyclohexyl halides. The reactions of 2 with 3 were carried out in solvents such as toluene, xylene and chlorobenzene at their boiling-point temperature in the presence of p-toluenesulfonic acid (Scheme 4, path e). Among these solvents, chlorobenzene was selected as the best since cyclohexylated product 4 was obtained in highest yield of 86%, while toluene and xylene provided 53% and 42% yields of 4, respectively, under the same set of reaction conditions. Reaction in chlorobenzene below 80 °C did not initiate conversion. When reaction of 2 with 3 was carried out without solvent and by using p-toluenesulfonic acid at 95 °C, the desired compound 4 was isolated in 38% yield.
By keeping chlorobenzene constant in the role of reaction solvent, further optimization was carried out to select an appropriate acid catalyst for this transformation. The reactions of 2 with 3 were carried out by using various acid catalysts such as p-toluenesulfonic acid (p-TSA), methanesulfonic acid (MSA), sulfuric acid, tungstate sulfuric acid (TSA) and phosphomolybdic acid; results are summarized in Table 1. Among the listed acids used, the p-toluenesulfonic acid-mediated cyclohexylation reaction afforded the highest yield of 4 in 86% (Table 1, entry 1), while the lowest yield was obtained from the tungstate sulfuric acid-mediated cyclohexylation reaction (Table 1, entry 4). Methanesulphonic acid-mediated cyclohexylation provided an average yield of 4 of 56% (Table 1, entry 2), while inferior yields were isolated from sulfuric acid – and phosphomolybdic acid – mediated cyclohexylation reactions (Table 1, entry 3 and 5, respectively).
While considering mechanistic aspects for the synthesis of 4, we assumed that the reaction could follow the general pathway of acid (p-TSA, in this case) mediated alkylation of naphthol and might be analogous to other acid-catalysed Friedel–Crafts alkylations.12d,e The cyclohexyl cation, generated through reactions between cyclohexanol 3 and p-TSA, would attack 1-naphthol 2, and was followed by rearomatisation to afford 2-cyclohexyl-1-naphthol, 4.
During the screening of reaction sequences for transforming 4 into 1, reaction conditions developed by Harrity et al.13 were tested and found to provide satisfactory results. Compound 4 was oxidized to 2-cyclohexyl-1,4-naphthoquinone 5 by using a mild oxidant such as 30% hydrogen peroxide in the presence of hydrochloric acid at room temperature and provided 68% yield of 5. Compound 5 was further converted into 2-cyclohexyl-(2,3)-oxirane-1,4-naphthoquinone, 6, (ref. 14) by using 30% hydrogen peroxide in the presence of aqueous sodium carbonate. The reaction smoothly occurred at room temperature and provided 76% yield of 6. Isolated epoxide intermediate 6 was then isomerized by using sulfuric acid at room temperature and the desired product 1 was isolated in good yield of 76% after work-up and purification.
Conclusion
The newly developed synthetic route of parvaquone has advantages of being operationally simple, environmentally benign and requires cheap and commercially accessible raw materials and reagents, which may contribute to cost reduction and thus make the process economically viable. The intermediates 4, 5 and 6 were obtained in good yields of 86%, 68% and 76%, respectively, leading to an overall yield of 33.8%. Use of 30% hydrogen peroxide, an industrially accepted green oxidant, under control conditions would make the developed process favourable for scaling up when compared with hazardous peroxides under drastic inert atmosphere conditions.Acknowledgements
PCP is grateful to the Technical Education Quality Improvement Program (TEQIP) for financial support given to this work.Notes and references
- (a) S. Patai, in The Chemistry of Quinonoid Compounds, ed. Z. Rappoport, Wiley, New York, 1988, vol. 2, parts 1 and 2 Search PubMed; (b) R. H. Thomson, in Naturally Occuring Quinones IV, Blackie Academic, London, 1997 Search PubMed; (c) R. P. Verma, Anti-Cancer Agents Med. Chem., 2006, 6, 489 CrossRef CAS and references cited therein; (d) S. Spyroudis, Molecules, 2000, 5, 1291 CrossRef CAS PubMed.
- L. F. Fieser and M. T. Leffler, US Pat., 2 553 648, 1951.
- R. Bishop, A. Musoke, S. Morzaria, M. Gardner and V. Nene, Parasitology, 2004, 129, 271 CrossRef.
- M. Mhadhbi, A. Naouach, A. Boumiza, M.-F. Chaabani, S. Ben- Abderazzak and M.-A. Darghouth, Vet. Parasitol., 2010, 169, 241 CrossRef CAS PubMed.
- N. McHardy, A. T. Hudson, D. W. Morgan, D. G. Rae and T. T. Dolan, Res. Vet. Sci., 1983, 35, 347 CAS.
- A. Erdemir, M. Aktas, N. Dumanli and D. Turgut-Balik, Vet. Med., 2012, 57, 559 CAS and references cited therein.
- L. F. Fieser, US Pat., 2 398 418, 1946.
- E. S. Huyser and B. Amini, J. Org. Chem., 1968, 33, 576 CrossRef CAS.
- B. S. Khambay and D. Batty, WO Pat., 9 621 354, 1996.
- H. Britton, D. Catterick, A. N. Dwyer, A. H. Gordon, S. G. Leach, C. McCormick, C. E. Mountain, A. Simpson, D. R. Stevens, M. W. J. Urquhart, C. E. Wade, J. Warren, N. F. Wooster and A. Zilliox, Org. Process Res. Dev., 2012, 16, 1607 CrossRef CAS.
- (a) C. R. Solorio-Alvarado, C. G. Rodriguez-Cendejas and E. Pena-Cabrera, ARKIVOC, 2003, 172 CrossRef CAS , (xi); (b) C. R. Solorio-Alvarado, C. Alvarez-Toledano and E. Peña-Cabrera, ARKIVOC, 2004, 64 CrossRef CAS , (i).
- (a) B. Alberti, Justus Liebigs Ann. Chem., 1926, 450, 304 CrossRef CAS; (b) R. P. Perkin, US Pat., 2 125 310, 1936; (c) S. P. Starkov, N. L. Polyanskaya, N. A. Vozhzhova and G. S. Leonova, Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol., 1977, 20, 1099 CAS; (d) T. Nishimura, S. Ohtaka, A. Kimura, E. Hayama, Y. Haseba, H. Takeuchi and S. Uemura, Appl. Catal., A, 2000, 194–195, 415 CrossRef CAS; (e) M. Rueping and B. J. Nachtsheim, Beilstein J. Org. Chem., 2010, 6, 6 Search PubMed.
- J. P. A. Harrity, W. J. Kerr, D. Middlemiss and J. S. Scott, J. Organomet. Chem., 1997, 532, 219 CrossRef CAS.
- L. F. Fieser and M. Fieser, J. Am. Chem. Soc., 1948, 70, 3177 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra09934f |
| ‡ Current address: Department of Chemistry, University of Louisville, Louisville, KY 40292, USA. |
| This journal is © The Royal Society of Chemistry 2014 |