Modified extra-large mesoporous silica supported Au–Ni as a highly efficient catalyst for oxidative coupling of aldehydes with methanol†
Junxing Hana,
Suojiang Zhang*a,
Juan Zhangb and
Ruiyi Yana
aState Key Laboratory of Multiphase Complex System, CAS Key Laboratory of Green Process and Engineering, Beijing Key Laboratory of Ionic Liquids Clean Process, Institute of Process Engineering, Chinese Academy of Sciences, Beijing, 100190, China. E-mail: sjzhang@ipe.ac.cn; Tel: +86-10-82544875
bCollege of Chemical and Pharmaceutical Engineering, Hebei University of Science and Technology, Shijiazhuang, 050018, China
First published on 23rd October 2014
Abstract
Herein, we report for the first time that a La–Mg composite oxide modified extra-large mesoporous FDU-12 supported Au–Ni bimetallic catalyst exhibits excellent performance for oxidative coupling of aldehydes (including benzyl and aliphatic aldehydes) with methanol to directly produce methyl esters.
Oxidative reactions are of academic and industrial importance for producing valuable chemicals and intermediates.1 Among them, oxidative coupling of aldehydes with methanol is a highly efficient route to directly synthesize methyl esters without the participation of carboxylic acids or carboxylic acid derivatives.2 One of the most representative examples is the oxidative coupling of methylacrolein (MAL) with methanol to produce methyl methacrylate (MMA), an important monomer for polymers. Traditionally, oxidative coupling of aldehydes with alcohols was conducted with stoichiometric amounts of toxic heavy metal salts such as KMnO4, CrO3, V2O5 or peroxovanadium,3 which is an environmentally unfriendly process. Catalytic oxidative coupling of aldehydes with methanol using molecular oxygen as the oxidant remains an urgent and challenging task.
Gold nanoparticle catalysts have attracted increasing interest due to their high efficiency in a wide range of oxidative reactions.4 However, just a few examples provide catalytic performance of supported gold catalysts for oxidative coupling of aldehydes with alcohols. Au/TiO2 showed high efficiency for the oxidative coupling of benzaldehyde with methanol but low activity for the transformation of aliphatic acrolein.5 Au–Ni bimetals supported on Al–Mg composite oxide modified silica provided high selectivity for the oxidative coupling of MAL with methanol, but the MAL conversion was merely 62%.6
Since Fan and co-workers found that FDU-12 supported gold nanoparticles exhibited high stability in aerobic oxidation of CO and benzaldehyde,7 ordered mesoporous FDU-12 has been identified as a promising support for oxidative reactions due to its unique structures such as extra-large entrance size and high pore volume. Herein, we describe for the first time that after modifying with La–Mg composite oxide, FDU-12 supported Au–Ni bimetallic catalyst exhibits high activity and stability for oxidative coupling of aldehydes (including benzyl and aliphatic aldehydes) with methanol to directly produce methyl esters.
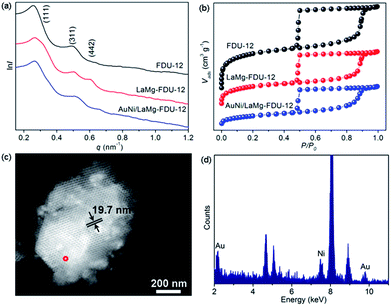
Ordered extra-large mesoporous FDU-12 was synthesized according to the literature method.8 Then, FDU-12 was impregnated with a mixture solution of La(NO3)3 and Mg(NO3)2. After calcination in air at 823 K, La–Mg composite oxide modified FDU-12 (LaMg–FDU-12) was prepared. Au and Ni precursors were introduced onto LaMg–FDU-12 simultaneously. After calcination in air at 723 K, AuNi/LaMg–FDU-12 catalyst was obtained. Small-angle X-ray scattering (SAXS) pattern of FDU-12 showed two peaks at q-values of 0.26 and 0.49 nm−1 assigned to (111) and (311) reflections of an ordered face-centered cubic mesostructure (Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) with a unit cell of 42.66 nm (Fig. 1a). LaMg–FDU-12 provided three scattering peaks in the SAXS pattern at q-values of 0.27, 0.50 and 0.60 nm−1 (Fig. 1a). The shift of q-values to higher positions could be attributed to the interaction between La–Mg composite oxides and FDU-12 pore walls, which resulted in a little shrinkage of FDU-12 framework. As a result, the d-spacing of (111) lattice decreased from 24.2 to 23.3 nm. No further shrinkage was observed during the following course of loading Au–Ni bimetals. SAXS pattern of AuNi/LaMg–FDU-12 resembles that of its mother LaMg–FDU-12, indicating that the FDU-12 framework was stable and the ordered extra-large mesopores of FDU-12 were preserved. Typical type-IV isomers with large H2 hysteresis loops could be seen for bare, modified and Au–Ni supported FDU-12 (Fig. 1b). The pore size of AuNi/LaMg–FDU-12 was 18.0 nm (Fig. S1, ESI†), which is very close to STEM observation results considering the thickness of pore walls (Fig. 1c). EDX profile clearly manifested the existence of Au and Ni in AuNi/LaMg–FDU-12 (Fig. 1d). Textural parameters of typical samples were summarized in Table S1 (ESI†). SAXS, N2-sorption, STEM and EDX experiments confirm the conclusion that Au–Ni bimetals have been successfully supported on the La–Mg composite oxide modified extra-large mesoporous FDU-12.
m) with a unit cell of 42.66 nm (Fig. 1a). LaMg–FDU-12 provided three scattering peaks in the SAXS pattern at q-values of 0.27, 0.50 and 0.60 nm−1 (Fig. 1a). The shift of q-values to higher positions could be attributed to the interaction between La–Mg composite oxides and FDU-12 pore walls, which resulted in a little shrinkage of FDU-12 framework. As a result, the d-spacing of (111) lattice decreased from 24.2 to 23.3 nm. No further shrinkage was observed during the following course of loading Au–Ni bimetals. SAXS pattern of AuNi/LaMg–FDU-12 resembles that of its mother LaMg–FDU-12, indicating that the FDU-12 framework was stable and the ordered extra-large mesopores of FDU-12 were preserved. Typical type-IV isomers with large H2 hysteresis loops could be seen for bare, modified and Au–Ni supported FDU-12 (Fig. 1b). The pore size of AuNi/LaMg–FDU-12 was 18.0 nm (Fig. S1, ESI†), which is very close to STEM observation results considering the thickness of pore walls (Fig. 1c). EDX profile clearly manifested the existence of Au and Ni in AuNi/LaMg–FDU-12 (Fig. 1d). Textural parameters of typical samples were summarized in Table S1 (ESI†). SAXS, N2-sorption, STEM and EDX experiments confirm the conclusion that Au–Ni bimetals have been successfully supported on the La–Mg composite oxide modified extra-large mesoporous FDU-12.
Oxidative coupling of MAL with methanol was firstly selected to evaluate the performance of AuNi/LaMg–FDU-12 catalyst and the results were listed in Table 1. Blank experiments showed that a MAL conversion of 38% was obtained in the absence of both catalysts and bases, while the MMA selectivity was negligible and the main by-product was 1,1-dimethoxy-2-methylpropylene. Adding Mg(OH)2 into the reaction system greatly inhibited the formation of 1,1-dimethoxy-2-methylpropylene, but the MAL conversion decreased to 6%. Both bare FDU-12 and LaMg–FDU-12 were inactive for the oxidative coupling of MAL with methanol. After loading Au–Ni bimetals, the prepared AuNi/LaMg–FDU-12 catalyst exhibited a high selectivity for MMA with a MAL conversion of 61%. As a comparison, unmodified AuNi/FDU-12 was ineffective for the reaction, because it is difficult for bare FDU-12 to adsorb Au and Ni precursors to form the so-called AuNi/FDU-12 catalyst. The loading amounts of Au and Ni on bare FDU-12 was negligible. We then tried to prepare AuNi/FDU-12 catalyst with the incipient wetness impregnation method to maintain the complete loading of Au and Ni precursors. However, the prepared AuNi/FDU-12(IWI) catalyst also showed poor performance. In XRD patterns, characteristic Au diffraction peaks appeared at 38.2, 44.3, 64.6, 77.4 and 81.8° for AuNi/FDU-12(IWI), indicating an average Au nanoparticle size of 55 nm (Fig. S2, ESI†). It is well known that large Au particles are inactive to catalyze reactions. No diffraction peaks belonging to Au or Ni were detected for AuNi/LaMg–FDU-12 catalyst due to small Au–Ni nanoparticle size (Fig. S3, ESI†). TEM images showed that Au–Ni nanoparticles uniformly dispersed on the La–Mg composite oxide modified FDU-12 with a particle size of 2.3 nm (Fig. 2a). CO2-TPD profiles manifested that no obvious CO2 desorption peaks were observed on bare FDU-12, while LaMg–FDU-12 provided two desorption peaks at 97 and 263 °C (Fig. 2b), indicating that modification of FDU-12 with La–Mg composite oxides enhanced the basic strength and amounts of basic sites, which promoted the dispersion of Au–Ni nanoparticles and greatly improved catalytic efficiency.
| Catalyst | T (°C) | Conversion (%) | Selectivity (%) |
|---|---|---|---|
| a Reaction conditions: catalyst 0.75 g, MAL 0.04 mol, methanol 0.64 mol, Mg(OH)2 as the base, oxygen 0.3 MPa, 30 mL min−1 of the oxygen flow rate, 2 h.b Without addition of Mg(OH)2.c Au and Ni precursors were supported onto bare FDU-12 with the incipient wetness impregnation method at room temperature. | |||
| Noneb | 60 | 38 | 0 |
| None | 60 | 6 | 0 |
| FDU-12 | 60 | 4 | 0 |
| LaMg–FDU-12 | 60 | 14 | 0 |
| AuNi/FDU-12 | 60 | 6 | 0 |
| AuNi/FDU-12 (IWI)c | 60 | 7 | 0 |
| AuNi/LaMg–FDU-12 | 60 | 61 | 94 |
| AuNi/LaMg–SiO2 | 60 | 36 | 61 |
| AuNi/AlMg–FDU-12 | 60 | 42 | 70 |
| AuNi/La–FDU-12 | 60 | 36 | 82 |
| AuNi/Mg–FDU-12 | 60 | 50 | 85 |
| AuNi/LaMg–FDU-12b | 60 | 56 | 94 |
| Au/LaMg–FDU-12 | 60 | 53 | 89 |
| Ni/LaMg–FDU-12 | 60 | 5.2 | 0 |
| AuNi/LaMg–FDU-12 | 70 | 74 | 94 |
| AuNi/LaMg–FDU-12 | 80 | 85 | 95 |
| AuNi/LaMg–FDU-12 | 90 | 97 | 96 |
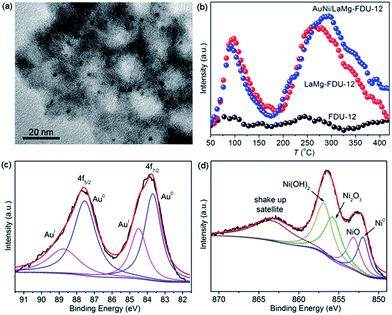 | ||
| Fig. 2 (a) TEM image of AuNi/LaMg–FDU-12; (b) CO2-TPD profiles of FDU-12, LaMg–FDU-12 and AuNi/LaMg–FDU-12; (c) XPS of Au 4f in AuNi/LaMg–FDU-12; (d) XPS of Ni 2p3/2 in AuNi/LaMg–FDU-12. | ||
We found that both MAL conversion and MMA selectivity decreased after substituting FDU-12 with silica. The reason could be attributed to the ordered extra-large mesopore structures of FDU-12, which could facilitate the diffusion of substrates and products. Meanwhile, AuNi/LaMg–FDU-12 showed much better performance than that of AuNi/AlMg–FDU-12. The latter provided a MAL conversion of 42% with a MMA selectivity of 70%.
Au–Ni bimetallic catalysts modified with La–Mg composite oxides showed superior performance to Au–Ni catalysts merely modified with La or Mg oxides. In the same way, Au–Ni bimetallic catalysts were more efficient than monometallic Au or Ni catalysts. XPS spectrum of Au 4f showed that after calcination AuIII precursors were mainly reduced to Au0 (83.7 and 87.6 eV) associated with partially reduced AuI species (84.5 and 88.7 eV) (Fig. 2c).9 The metallic Au0 was identified to be active for oxidative coupling of aldehydes with methanol.2 Correspondingly, in the Ni 2p3/2 region, besides Ni0 (852.0 eV), NiO (853.1 eV) and Ni(OH)2 (856.9 eV), a fraction of NiII precursors were oxidized to high-valence Ni2O3 (855.7 eV) (Fig. 2d).10 Suzuki and co-workers reported that high-valence nickel oxides were also effective for oxidative coupling of aldehydes with methanol.6 Therefore, the synergetic effect between Au and Ni made the bimetallic AuNi/LaMg–FDU-12 catalyst more efficient than monometallic counterparts. To our delight, AuNi/LaMg–FDU-12 catalyst offered a MAL conversion of 56% with the MMA selectivity of 94% even in the absence of bases.
The reaction temperature affected catalytic performance greatly. When the reaction temperature was elevated to 90 °C, a MAL conversion of 97% with a MMA selectivity of 96% was acquired.
Besides MAL, we also selected variety of benzyl and aliphatic aldehydes as substrates to investigate the catalytic performance of AuNi/LaMg–FDU-12 catalyst and the results were summarized in Table 2. With Mg(OH)2 as a weak base, AuNi/LaMg–FDU-12 showed high activity and selectivity for oxidative coupling of propionaldehyde, isobutyraldehyde and methylacrolein with methanol. Compared with saturated aliphatic aldehydes, the conversion of unsaturated aliphatic aldehyde, methylacrolein, dropped from 100% to 97% with the selectivity increasing to 96%. Friend and co-workers demonstrated that oxidative coupling of aldehydes with methanol started with deprotonation of methanol, the formed methoxy then attacked the aldehydic carbon atoms to form hemiacetal intermediates and finally further β-H elimination of hemiacetal yielded methyl esters.2 Based on this finding, the conjugated effect between C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of unsaturated aldehydes passivated carbonyl groups, which made the nucleophilic attack of methoxy difficult. Consequently, the conversion of unsaturated aldehydes dropped.
O of unsaturated aldehydes passivated carbonyl groups, which made the nucleophilic attack of methoxy difficult. Consequently, the conversion of unsaturated aldehydes dropped.
| Entry | Substrate | Product | Conversion (%) | Selectivity (%) |
|---|---|---|---|---|
| a Reaction conditions: catalyst 0.75 g, substrate 0.04 mol, methanol 0.64 mol, 0.3 MPa of oxygen, 30 mL min−1 of the oxygen flow rate.b Reaction time 2 h, Mg(OH)2 as the base, 0.05 g.c Reaction time 8 h, K2CO3 as the base, 0.1 g.d Reaction time 16 h, K2CO3 as the base, 0.1 g. | ||||
| 1b | 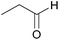 |
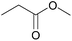 |
100 | 90 |
| 2b | 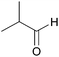 |
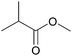 |
100 | 94 |
| 3b | 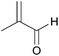 |
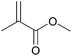 |
97 | 96 |
| 4c | 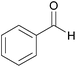 |
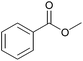 |
100 | 94 |
| 5c | 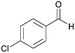 |
 |
96 | 93 |
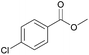
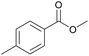
| 6c | 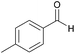 |
 |
100 | 96 |
| 7c | 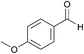 |
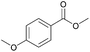 |
100 | 99 |
| 8c | 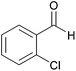 |
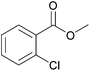 |
70 | 80 |
| 9c | 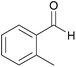 |
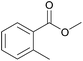 |
100 | 93 |
| 10c | 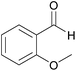 |
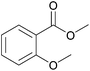 |
100 | 99 |
| 11c | 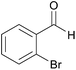 |
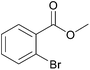 |
74 | 89 |
| 12d | 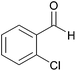 |
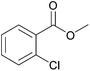 |
97 | 87 |
Compared with aliphatic aldehydes, longer reaction time and stronger base are needed for oxidative coupling of benzyl aldehydes due to the conjugated effect between benzene ring and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. In the presence of K2CO3, the conversion and the selectivity of benzaldehyde were as high as 100% and 94%, respectively. Complete conversion with selectivity over 90% was achieved for benzyl aldehydes containing electron-donating groups such as CH3 and OCH3, no matter where the substituents located. For benzyl aldehydes containing electron-withdrawing groups such as Cl and Br, the position of substituents had an important effect. With 4-chlorobenzaldehyde as the substrate, the conversion and the selectivity of 96% and 93% were obtained, which were very close to those of benzaldehyde. However, for 2-chlorobenzaldehyde and 2-bromobenzaldehyde, conversions dropped to 70% and 74%, respectively, which meant that electron-withdrawing groups located at ortho-position passivated the aldehyde groups more. By extending reaction time to 16 h, the conversion of 2-chlorobenzaldehyde increased to 97% with a methyl ester selectivity of 87%. In a word, AuNi/LaMg–FDU-12 showed high efficiency for oxidative coupling of aldehydes with methanol.
O. In the presence of K2CO3, the conversion and the selectivity of benzaldehyde were as high as 100% and 94%, respectively. Complete conversion with selectivity over 90% was achieved for benzyl aldehydes containing electron-donating groups such as CH3 and OCH3, no matter where the substituents located. For benzyl aldehydes containing electron-withdrawing groups such as Cl and Br, the position of substituents had an important effect. With 4-chlorobenzaldehyde as the substrate, the conversion and the selectivity of 96% and 93% were obtained, which were very close to those of benzaldehyde. However, for 2-chlorobenzaldehyde and 2-bromobenzaldehyde, conversions dropped to 70% and 74%, respectively, which meant that electron-withdrawing groups located at ortho-position passivated the aldehyde groups more. By extending reaction time to 16 h, the conversion of 2-chlorobenzaldehyde increased to 97% with a methyl ester selectivity of 87%. In a word, AuNi/LaMg–FDU-12 showed high efficiency for oxidative coupling of aldehydes with methanol.
Furthermore, AuNi/LaMg–FDU-12 showed high stability for the oxidative coupling of methylacrolein with methanol. No deactivation was observed after recycling for six times (Fig. S7, ESI†). The yield of methyl methacrylate maintained at 94%. Ordered extra-large mesopores of FDU-12 were persisted and no diffraction peaks belonging to Au or Ni could be detected (Fig. S8–S10, ESI†).
Conclusions
In conclusion, ordered extra-large mesopores of FDU-12, enhanced alkalinity by La–Mg composite oxide and synergetic effect between Au and Ni provided AuNi/LaMg–FDU-12 excellent performance for oxidative coupling of aldehydes with methanol to directly produce methyl esters. The catalyst will be a promising alternative to the conventional Pd–Pb catalysts which cause serious Pb contamination to the environment.Acknowledgements
This work was supported by the National Key Basic Research Program of China (2015CB251401), the National Natural Science Foundation of China (21306203) and the National High-Tech Research and Development Program of China (2012AA062903).Notes and references
- (a) L. Kesavan, R. Tiruvalam, M. H. A. Rahim, M. I. Saiman, D. I. Enache, R. L. Jenkins, N. Dimitratos, J. A. Lopez-Sanchez, S. H. Taylor, D. W. Knight, C. J. Kiely and G. J. Hutchings, Science, 2011, 331, 195–199 CrossRef CAS PubMed; (b) B. N. Zope, D. D. Hibbitts, M. Neurock and R. J. Davis, Science, 2010, 330, 74–78 CrossRef CAS PubMed.
- B. J. Xu, X. Y. Liu, J. Haubrich, R. J. Madix and C. M. Friend, Nat. Chem., 2010, 2, 61–65 CrossRef CAS PubMed.
- (a) K. Ekoue-Kovi and C. Wolf, Chem.–Eur. J., 2008, 14, 6302–6315 CrossRef CAS PubMed; (b) R. Gopinath, B. Barkakaty, B. Talukdar and B. K. Patel, J. Org. Chem., 2003, 68, 2944–2947 CrossRef CAS PubMed; (c) B. R. Travis, M. Sivakumar, G. O. Hollist and B. Borhan, Org. Lett., 2003, 5, 1031–1034 CrossRef CAS PubMed.
- M. Haruta, Angew. Chem., Int. Ed., 2014, 53, 52–56 CrossRef CAS PubMed.
- C. Marsden, E. Taarning, D. Hansen, L. Johansen, S. K. Klitgaard, K. Egeblad and C. H. Christensen, Green Chem., 2008, 10, 168–170 RSC.
- K. Suzuki, T. Yamaguchi, K. Matsushita, C. Iitsuka, J. Miuya, T. Akaogi and H. Ishida, ACS Catal., 2013, 3, 1845–1849 CrossRef CAS.
- (a) G. C. Ma, X. Q. Yan, Y. L. Li, L. P. Xiao, Z. J. Huang, Y. P. Lu and J. Fan, J. Am. Chem. Soc., 2010, 132, 9596–9597 CrossRef CAS PubMed; (b) G. C. Ma, A. Binder, M. F. Chi, C. Liu, R. C. Jin, D. E. Jiang, J. Fan and S. Dai, Chem. Commun., 2012, 48, 11413–11415 RSC.
- (a) J. Fan, C. Z. Yu, F. Gao, J. Lei, B. Z. Tian, L. M. Wang, Q. Luo, B. Tu, W. Z. Zhou and D. Y. Zhao, Angew. Chem., Int. Ed., 2003, 42, 3146–3150 CrossRef CAS PubMed; (b) X. Q. Yan, X. J. Wang, Y. Tang, G. C. Ma, S. H. Zou, R. H. Li, X. G. Peng, S. Dai and J. Fan, Chem. Mater., 2013, 25, 1556–1563 CrossRef CAS.
- H. Kitagawa, N. Kojima and T. Nakajima, J. Chem. Soc., Dalton Trans., 1991, 3121–3125 RSC.
- I. Kosc, I. Hotovy, T. Roch, T. Plecenik, M. Gregor, M. Predanocy, M. Cehlarova, P. Kus and A. Plecenik, Appl. Surf. Sci., 2014, 312, 120–125 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and extra tables and figures. See DOI: 10.1039/c4ra09923k |
| This journal is © The Royal Society of Chemistry 2014 |