Base-catalysed hydrolysis of PIM-1: amide versus carboxylate formation†
Abstract
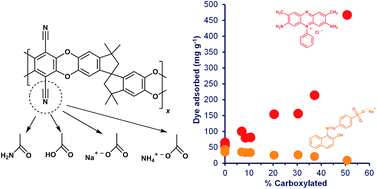
Chemical modification can be used to tailor the properties of PIM-1, the prototypical polymer of intrinsic microporosity, which shows promise for applications such as membrane and adsorption processes for gas and liquid separations. Base-catalysed hydrolysis of PIM-1 has previously been assumed to yield only carboxylated products. In this work, hydrolysis was carried out at 120 °C with 20% NaOH and at 100 °C with 10% NaOH in a water–ethanol mixture, and a combination of IR, UV, 1H NMR and elemental analysis was used to demonstrate that the hydrolysis products contain a mixture of amide, carboxylic acid, ammonium carboxylate and sodium carboxylate structures. The amide-PIM-1 structure has not previously been reported. Even the most fully hydrolysed samples had a substantial proportion of amide, with most samples being >50% amide. On hydrolysis there was a decrease in the water contact angle (from 85° for PIM-1 to about 60° for the most fully hydrolysed samples) and a decrease in the BET surface area. The adsorption of dyes from aqueous solution was shown to depend on the composition of the polymer. Uptake of the cationic dye Safranin O increased dramatically with increasing percentage carboxylation, the most highly carboxylated sample showing 31 times the uptake of the parent polymer, whereas uptake of the anionic dye Orange II decreased with increasing percentage carboxylation.

 Please wait while we load your content...
Please wait while we load your content...