Ameliorating acidic soil using bioelectrochemistry systems†
Abstract
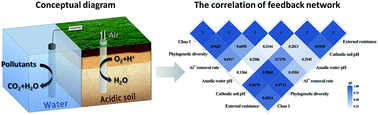
Soil acidification has been a threat to sustainable agricultural development as a global environmental problem. The bioelectrochemistry system (BES) is an eco-friendly and low-cost technique for ameliorating acidic soil when compared with traditional technologies. Here, operational parameters from soil load and external resistance were investigated through mapping the pH changes of the soil. The result showed that approximately a quarter of the soil load in the operating unit was an optimized choice based on the performance at the unit and overall levels. The lower external resistance was more conducive to enhance the electric force for the increase of the soil pH. This study not only proved the feasibility of BES for ameliorating acidic soil, but also comprehensively described the feedback network of soil acidity, Al3+ removal and microbial community. After BES-amelioration, the exchangeable Al3+ obviously reduced and the microbial community structure shifted. The exploration of operational parameters and a feedback network provided theoretical support for the application of BES-based technologies in reclaiming soil acidity.

 Please wait while we load your content...
Please wait while we load your content...