Filipins: the first antifungal “weed killers” identified from bacteria isolated from the trap-ant†
Hong Gaoab,
Sabine Grüschowab,
Jörg Barkec,
Ryan F. Seipkec,
Lionel M. Hilld,
Jérôme Orivele,
Douglas W. Yucf,
Matthew Hutchingsc and
Rebecca J. M. Goss*ab
aSchool of Chemistry, University of St. Andrews, St. Andrews, Fife, UK KY16 9ST. E-mail: rjmg@st-andrews.ac.uk
bSchool of Chemistry, University of East Anglia, Norwich Research Park, Norwich, Norfolk, UK NR4 7TJ
cSchool of Biological Sciences, University of East Anglia, Norwich Research Park, Norwich, Norfolk, UK NR4 7TJ
dJohn Innes Centre, Norwich Research Park, Norwich, UK NR4 7UH
eCNRS, UMR Ecologie des Forêts de Guyane, Campus Agronomique, BP 316, 97379 Kourou Cedex, France
fState Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Kunming, Yunnan, P. R. China 650223
First published on 27th October 2014
Abstract
Allomerus ants ensure that they have sufficient nitrogen in their diet by trapping and consuming other insects. In order to construct their traps, like the more extensively studied leaf cutter ants, they employ fungal farming. Pest management within these fungal cultures has been speculated to be due to the ants' usage of actinomycetes capable of producing antifungal compounds, analogous to the leafcutter ant mutualism. Here we report the first identification of a series of antifungal compounds, the filipins, and their associated biosynthetic genes isolated from a bacterium associated with this system.
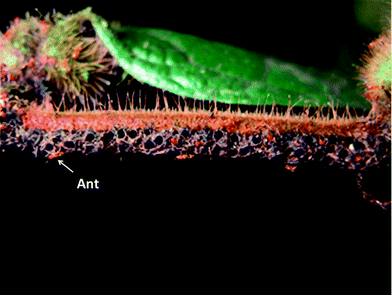
Allomerus (Myrmicinae) ants construct elaborate galleried traps on the stems of their specialist host plants, gluing lengths of cut plant hairs (trichomes) across “pillars” of remaining uncut plant hairs and reinforcing the structure with mycelia of purpose-cultured fungi (Fig. 1). These galleried traps contain regularly spaced holes just large enough for these tiny red ants to stick their heads through. When an insect alights upon the structure, the ants grab the legs to immobilize the prey and stretch it out across the galleried floor, repeatedly stinging it, before moving it to a leaf pouch for dissection.1 Only mycelia from a single species of sooty mould fungus, from the order Chaetothyriales, have so far been observed in this mutualism; though spores from up to forty-five other fungal species have been detected in the fungal traps and ants' waste, suggesting that the ants are active in maintaining their fungal monoculture.2
The more extensively studied leafcutter ant system has revealed that the leafcutter ants live in mutualism with bacteria that are capable of generating cocktails of antifungal compounds that, in addition to mechanical maintenance and weeding by the ants, are utilized to maintain a monoculture fungal garden free from parasites and less advantageous fungal species.3–5 Key antifungal compounds utilized by the leafcutter ants include candicidin, nystatin-like compounds3 and antimycin.6 There is urgent need for new compounds for the treatment of drug-resistant fungal pathogens. The ant/fungal/bacterial mutualism systems could potentially yield new and useful compounds.
Recently we isolated and identified seven culturable bacteria (six Streptomyces and one Amycolatopsis) from the cuticles of worker ants of the species Allomerus decemarticulatus and Allomerus octoarticulatus and demonstrated that laboratory cultures of four of these strains (FG22, FG23, FG25 and FG26) exhibited antifungal activity.7 We report here the first identification of a family of antifungal compounds from the trap ant system.
The four bacterial strains were cultured in triplicate in a panel of twelve media with varying carbon and nitrogen sources (see ESI, Table S1†), and concentrated entire culture broths as well as extracts assayed against the fungus Candida albicans. Ethyl acetate extracts of one Streptomyces strain, FG26, cultured in M9, a medium containing soluble starch as the sole carbon source, showed potent activity against C. albicans. The 16S rDNA sequence of FG26 indicated that the strain was closest to Streptomyces misionensis type strain NRRL B-3230 (100% identity),7 a little studied strain from Argentina.8 S. misionensis had been reported to produce the antifungal tetraene BH890 (C41H74NO16, mass = 837.033; structure unknown),9 and we initially postulated that this compound might be responsible for the potent antifungal activity that we observed. However, we were unable to detect this compound in any of our culture conditions.
Careful activity-guided fractionation of our culture extract revealed the presence of a series of compounds with potent antifungal activity. LC-MSMS analysis was consistent with these compounds being three components of the filipin complex (Sigma-Aldrich, St. Louis, MO, USA), a mixture of related compounds, filipins I to IV (Fig. 2). The major filipin that was detected in the FG26 extract corresponds to filipin III by accurate mass (calculated for C35H58O11Na [M + Na]+ 677.3871, found 677.3872, error 0.1 ppm, Fig. S1 and S2†), fragmentation pattern (Fig. S6†), co-elution with the commercial standard filipin complex (Fig. S5†) and UV spectrum (Fig. S7†). Similarly, two minor metabolites consistent with filipins II (calculated for C35H58O10Na [M + Na]+ 661.3922, found 661.3922, error −0.01 ppm, Fig. S4†) and IV (calculated for C35H58O11Na [M + Na]+ 677.3871, found 677.3871, error −0.01 ppm, Fig. S3†) were also identified. The relative retention times of filipins II (20.9 min), III (18.0 min), and IV (18.9 min) produced by FG26 match those previously reported,10 and these data in combination with detailed gene sequence analysis are strongly indicative that these compounds are identical. The Argentinian S. misionensis had also been reported to generate a further antifungal, misionin, for which there was little characterisation. Published UV absorbance data for this compound, however, would be consistent with it being a filipin analogue.8
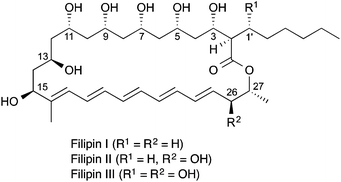 | ||
| Fig. 2 Filipin complex. Filipin IV is thought to be an epimer of filipin III at the 1′ or 3 position.11 | ||
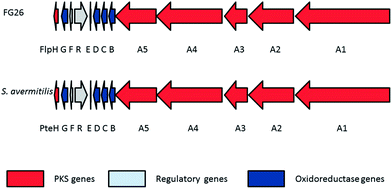
To further verify the identification of the first antifungals from a bacterium isolated from the Allomerus system, we sought the genes responsible for the biosynthesis of these compounds. Filipin biosynthesis has previously been reported in Streptomyces avermitilis, and the genes encoding the biosynthetic pathway identified.12 Investigation of the FG26 genome sequence revealed a biosynthetic cluster, with four open reading frames encoding modular polyketide synthases with a very high level of similarity to the genes previously identified as encoding filipin biosynthesis, supporting our findings that the bacterium produced filipin complex.
The filipin gene cluster identified from FG26 has the same ordering as that of S. avermitilis (Fig. 3). Both gene clusters encode 13 proteins, and their functions and identities are illustrated in Table 1. Sequence identities at the protein level are high, ranging from 83–92%. The identification of the filipin gene cluster supports the proposed ability of FG26 to produce filipins.
| FG26 protein | Proposed function | Streptomyces avermitilis MA-4680 protein | Protein identity (%) |
|---|---|---|---|
| FlpA1 | PKS | PteA1 | 84 |
| FlpA2 | PKS | PteA2 | 85 |
| FlpA3 | PKS | PteA3 | 84 |
| FlpA4 | PKS | PteA4 | 84 |
| FlpA5 | PKS | PteA5 | 85 |
| FlpB | Putative dehydrogenase | PteB | 92 |
| FlpC | Cytochrome P450 hydroxylase | PteC | 91 |
| FlpD | Cytochrome P450 hydroxylase | PteD | 88 |
| FlpE | Ferredoxin | PteE | 87 |
| FlpR | DnrI/RedD/AfsR-family transcriptional regulator | PteR | 78 |
| FlpF | LuxR family transcriptional regulator | PteF | 88 |
| FlpG | Putative oxidase | PteG | 92 |
| FlpH | Thioesterase | PteH | 80 |
In modular PKSs, the KR (ketoreductase) domains are responsible for the stereospecific formation of β-hydroxyacyl thioester intermediates.13 Bioinformatic analysis of KR domains has shown that conserved signature motifs correlate with the stereochemistry of the alcohol, and have thus been classified into A-type or B-type KR domains (Fig. 4).14 Comparative examination of these signature motifs in the newly identified FG26 filipin KR domains with those of the S. avermitilis homologues was indicative that the FG26 filipins and the S. avermitilis filipins shared the same stereochemistry. Of the 13 KR domains in the filipin biosynthetic gene cluster, KR1 and KR7–KR13 are responsible for the alcohol stereochemistry at positions 27, 15, 13, 11, 9, 7, 5, and 3, respectively (Fig. 2). KR1 and KR7 have the conserved LDD motif of B-type KR domains, whereas the remaining six KR domains lack the LDD motif but contain the conserved tryptophan in the second motif indicative of A-type KR domains (Fig. 4B). These assignments match the observed stereochemistry in filipins I–III; most importantly, they perfectly match the KR types of the S. avermitilis filipin PKS.15 The 26- and 1′-OH groups in filipin III are installed by the P450 enzymes PteC and PteD, respectively. PteC has been crystallised with its substrate filipin I.16 Structural alignment of PteC and the FG26 homologue FlpC shows all substrate binding residues to be conserved (Fig. S9†), which is a strong indication that the substrate positioning in FlpC and the stereochemical outcome of the hydroxylation will be identical to PteC. The sequence identity between PteD and FlpD is somewhat lower than that for the PteC/FlpC pair (88 vs. 91%, respectively) and no ligand-bound structure is available for PteD.16 This makes the prediction of substrate binding for FlpD less reliable. However, given the high sequence identity of all filipin biosynthetic genes and the conserved organisation of the biosynthetic cluster compared to S. avermitilis, in combination with LC-MSMS data and known shifts in retention times, even for filipin stereomers (filipin III and filipin IV), the assignment of the prevalent filipin produced by the ant symbiont FG26 as filipin III is very likely.
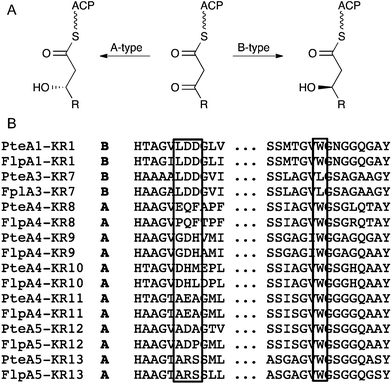 | ||
| Fig. 4 KR domain specificity. A: stereochemical outcome of A- and B-type KR domains. B: signature motifs of KR domains in the filipin PKS from FG26 (Flp) and S. avermitilis (Pte); diagnostic residues are boxed, the complete sequence alignment is provided in Fig. S8.† | ||
Conclusions
Several antifungal compounds have now been reported from symbiont actinomycetes of the leafcutting ants Acromyrmex, including polyene antibiotics such as candicidin6,17 and nystatin P13, and other antibiotics such as antimycin,6,18 actinomycin and valinomycin.16 Few investigations have so far been carried out of any other ant-fungus symbioses. Here we report initial investigations into identifying compounds associated with the ant Allomerus. In this work we reveal that the Allomerus-associated actinomycete, Streptomyces FG26, is capable of generating a suite of filipins. Further in vivo experimental work will be needed to test the hypothesis that the filipins produced by this putative ant symbiont indeed confer a fitness benefit on Allomerus.Acknowledgements
We acknowledge the support from the Marie Curie International Incoming Fellowship Grant within the 7th European Community Framework Programme (Grant no. 274110), and the European Commission (Seventh Framework Programme, Collaborative project “BlueGenics”, Grant no. 311848). This work has also benefited from an “Investissement d'Avenir” grant managed by the Agence Nationale de la Recherche (CEBA, ANR-10-LABX-25-01).Notes and references
- A. Dejean, P. J. Solano, J. Ayroles, B. Corbara and J. Orivel, Nature, 2005, 434, 973 CrossRef CAS PubMed.
- M. X. Ruiz-González, P. J. Malé, C. Leroy, A. Dejean, H. Gryta, P. Jargeat, A. Quilichini and J. Orivel, Biol. Lett., 2011, 7, 475 CrossRef PubMed.
- J. Barke, R. F. Seipke, S. Grüschow, D. Heavens, N. Drou, M. J. Bibb, R. J. M. Goss, D. W. Yu and M. I. Hutchings, BMC Biol., 2010, 8, 109 CrossRef PubMed.
- C. R. Currie, Annu. Rev. Microbiol., 2001, 55, 357 CrossRef CAS PubMed.
- R. Sen, H. D. Ishak, D. Estrada, S. E. Dowd, E. Hong and U. G. Mueller, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 17805 CrossRef CAS PubMed.
- R. F. Seipke, J. Barke, C. Brearley, L. Hill, D. W. Yu, R. J. M. Goss and M. I. Hutchings, PLoS One, 2011, 6, e22028 CAS.
- R. F. Seipke, J. Barke, M. X. Ruiz-Gonzalez, J. Orivel, D. W. Yu and M. I. Hutchings, Antonie van Leeuwenhoek, 2012, 101, 443 CrossRef CAS PubMed.
- A. P. Cercos, B. I. Eilberg, J. G. Goyena, J. Souto, E. E. Vautier and I. Widuczynski, Rev. Invest. Agric., 1962, 17, 5 Search PubMed.
- J. Martin and J. N. Porter, US Pat. 3,700,769, October 24, 1972.
- P. M. Kelly and K. A. Nabinger, J. Antibiot., 1985, 38, 485 CrossRef CAS.
- R. C. Pandey, N. Narasimhachari, K. L. Rinehart Jr and D. S. Millington, J. Am. Chem. Soc., 1972, 94, 4306 CrossRef CAS.
- H. Ikeda, K. Shin-ya and S. Omura, J. Ind. Microbiol. Biotechnol., 2014, 41, 233 CrossRef CAS PubMed.
- Y. Yin, R. Gokhale, C. Khosla and D. E. Cane, Bioorg. Med. Chem. Lett., 2001, 11, 1477 CrossRef CAS.
- P. Caffrey, ChemBioChem, 2003, 4, 654 CrossRef CAS PubMed.
- H. Ikeda, S. Kazuo and O. Satoshi, J. Ind. Microbiol. Biotechnol., 2014, 41, 233 CrossRef CAS PubMed.
- L. H. Xu, S. Fushinobu, S. Takamatsu, T. Wakagi, H. Ikeda and H. Shoun, J. Biol. Chem., 2010, 285, 16844 CrossRef CAS PubMed.
- S. Haeder, R. Wirth, H. Herz and D. Spiteller, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 4742 CrossRef CAS PubMed.
- I. Schoenian, M. Spiteller, M. Ghaste, R. Wirth, H. Herz and D. Spiteller, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 1955 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Culturing conditions, extractions, bioassays and LC-MS analysis are reported here. See DOI: 10.1039/c4ra09875g |
| This journal is © The Royal Society of Chemistry 2014 |