The effect of hydroxytyrosol and its nitroderivatives on catechol-O-methyl transferase activity in rat striatal tissue
Abstract
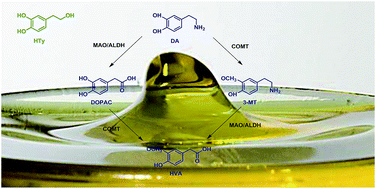
Hydroxytyrosol is a well-known phenolic compound with antioxidant properties that is found in virgin olive oil. Studies have shown that virgin olive oil has neuroprotective effects in rats; thus the purpose of the present study was to investigate the neuroprotective effect of hydroxytyrosol in rats. Additionally, this study aimed to investigate the neuroprotective potential of a homologous series of compounds with better lipophilic profiles in order to increase the assortment of compounds with a putative effect against Parkinson's disease (PD). In this context, the inhibition of catechol-O-methyl transferase (COMT) activity by hydroxytyrosol, nitrohydroxytyrosol, nitrohydroxytyrosol acetate and ethyl nitrohydroxytyrosol ether was investigated by measuring intracellular dopamine and its metabolite levels in the corpus striatum by high performance liquid chromatography (HPLC) with electrochemical detection. The animals received an acute (single dose; 20 mg kg−1; i.p.) or chronic (one daily dose for 5 days; 20 mg kg−1; i.p.) treatment of hydroxytyrosol and its nitroderivatives. For comparison, a commercial COMT inhibitor, Ro 41-0960, was also included. Our data show that acute and chronic systemic administration of these compounds produced a clear and statistically significant increase in the intracellular levels of dopamine and its metabolite, 3,4-dihydroxyphenylacetic acid. The increase in dopamine levels was very similar to the increase seen with Ro 41-0960 treatment. The effect of chronic treatment was stronger than that of acute treatment. With respect to the intracellular level of homovanillic acid, Ro 41-0960 produced a statistically significant decrease which it was not observed when hydroxytyrosol and its nitroderivatives were systemically administered. However, the chronic homovanillic acid treatment effect was stronger than the acute treatment. The results suggest that these compounds could inhibit COMT activity.

 Please wait while we load your content...
Please wait while we load your content...