DOI:
10.1039/C4RA09870F
(Paper)
RSC Adv., 2015,
5, 7697-7712
Solubilization and co-solubilization of carbamazepine and nifedipine in mixed micellar systems: insights from surface tension, electronic absorption, fluorescence and HPLC measurements
Received
5th September 2014
, Accepted 3rd December 2014
First published on 4th December 2014
Abstract
UV absorption spectral and HPLC study on the solubilization and co-solubilization behavior of antiepileptic drug Carbamazepine (CBZ) and calcium channel blocker Nifedipine (NFD), which are reported to have a synergistic potentiation, was carried out in sodium cholate based binary and ternary mixed micellar systems with non-ionic polysorbate (Tween20, Tween40) and polyoxyethylene (Brij30, Brij35, Brij56 and Brij58) surfactants. The surfactant–surfactant interactions and their effect on the aggregation number, solubility of drugs, solubilization site, surfactant–drug interactions and drug–drug interactions were evaluated and explained. Synergism in mixed micellization increases the aggregation number and decreases the polarity of palisade layer resulting in enhancement of core solubilization of drugs with concomitant decrease in palisade layer solubilization. In the C12 series, CBZ shows a decrease in solubility upon surfactant mixing, indicating an appreciable solubilization in the palisade layer, whereas in the C16 series an increase in its solubility was observed. For NFD, a decrease in solubility follows the trend of synergism in mixed micellization, which is more for strongly interacting surfactant systems. During co-solubilization, because CBZ occupies preferentially the palisade layer, its solubility is decreased and the solubilization of NFD, which mainly occurs within the micellar core, is favored. The magnitude of drug–drug interactions increases in mixed micelles and is more for the surfactant systems, showing more synergism in the mixed micelle formation. The mixed micellar media used in the present study, being biocompatible, are expected to be employed as solubilization and drug delivery vehicles for co-administration of these two drugs in vivo.
Introduction
Poor aqueous solubility paired with poor bioavailability of active pharmaceutical ingredients is a major challenge in the pharmaceutical industry. These solubility problems led to the development of application vehicles, such as mixed micelles, a demanding research topic in pharmaceutical technology. Valium MM and Konakion MM are two mixed micelles based formulations that are currently available in the pharmaceutical market.1 Mixed micelles usually have diameters less than 60 nm, which prevent their uptake by the reticuloendothelial system (RES) and consequently increases their in vivo circulation and facilitates their extravasation in sites with leaky vasculature such as tumors.2 The known classical mixed micellar systems are composed of bile salts and phospholipids but their fabrication involves the use of organic solvents, such as chloroform and methanol, which are required for solubilization of phospholipids.3 Thus, there arises a need to develop alternative mixed micelle formulations using components with good pharmaceutical acceptability. Bile salts are physiologically relevant, biocompatible and biodegradable molecules derived from cholesterol and can undergo aggregation in aqueous solutions.4 These are very safe and effective vehicles for medical applications and have been used in the solubilization of many poorly water soluble drugs such as griseofulvin,5 glutethimide,5 digoxin,6 leukotriene-D4 antagonists,7 and gemfibrozil8 and also as a delivery system for many other drugs and vitamins.9,10 The ability of bile salts to enhance the oral bioavailability of poorly water-soluble drugs has been recognized.11–13 They normally enhance the transport of lipophilic drugs across biological membranes, and hence enhance oral bioavailability.14–16 Moreover, such micellar systems are known to improve the solubility of extremely lipophilic drugs.17–19 Therefore, bile salts micelles and derived mixed systems are intensively investigated as drug carrier systems.20–22 In addition, from an economic or commercial perspective, this technique of solubilizing drugs within micellar media simplifies the manufacturing process and allows for large-scale production of drugs.
Polysorbates (commonly known as Tween surfactants) are nonionic surfactants that are very effective in solubilizing drugs23 and are used in the manufacture of a variety of pharmaceutical products.24 They are known to enhance the permeability of phospholipid membranes causing leakage of low molecular mass compounds.24 They induce alterations in the physicochemical properties of biomembranes and specifically increase the permeability of the sarcoplasmic reticulum.25 Alkyl polyoxyethylene ether (Brij) surfactants have also been extensively studied in pharmaceutical systems26–28 due to their minimum toxicity.
Carbamazepine (CBZ) is an antiepileptic drug used in the treatment of epilepsy, trigeminal neuralgia and bipolar disorders, and Nifedipine (NFD) is a calcium channel blocker used for the treatment of hypertension and angina pectoris.29 CBZ and NFD have low aqueous solubility and hence irregular and delayed absorption. An abnormal and excessive hypersynchronous firing within a group of epileptic neurons in the brain results in sudden alteration in motor activity and behavior.30–32 About 30% of the people with epilepsy have seizures that do not respond satisfactorily to the conventional antiepileptic drugs (CAEDs).33 These limitations with the CAEDs highlight the need for exploring drugs that could potentiate their action to make the treatment of epilepsy more effective. Medevite et al. have shown the presence of specific binding sites of calcium channel blockers (CCBs) that enable CAEDs to cross the blood brain barrier.34 Desmedt et al. reported that CCBs like cinnarizine and flunarizine have anticonvulsive properties in rats and mice.35 Rational polytherapy concept is based on the assumption that combining some antiepileptic drugs may results in supra-additive (synergistic) efficacy and infra additive (antagonistic) toxicity, resulting in an enhanced efficacy/toxicity profile. CCBs having antiepileptic properties were combined with established antiepileptic drugs. Flunarizine, a CCB, was given along with antiepileptic drugs as an add-on therapy and has been found to reduce seizure significantly,36 and nifedipine (NFD) was given along with carbamazepine (CBZ) to provide superior seizures control in maximal electroshock (MES)-induced and pentylenetetrazole (PTZ)-induced convulsions.37 There are reports where a patient with classical pattern of bipolar disorder with cycles of mania and depression responds to a combination of CBZ and NFD.38
In our earlier work29 we demonstrated that the single and simultaneous solubilization of CBZ and NFD in a single surfactant based micellar media is highly sensitive to the hydrophilic–lipophilic balance (HLB) value and the concentration of surfactants. The present study aims to enhance the micellar solubilization/co-solubilization of these drugs with simultaneous reduction in the amount of surfactant used by employing technologically more efficient surfactant mixtures. The solubilization/co-solubilization of drugs in sodium cholate based binary and ternary mixed micellar systems has not been reported. Therefore, as a part of our previous and extensive work29,39 on the micelle mediated solubilization of pharmaceutically active molecules, we present here the solubilization and co-solubilization of CBZ and NFD in biocompatible binary and ternary mixed micellar systems based on sodium cholate (Bile Salt), alkyl polyoxyethylene and alkyl polysorbate surfactants. In general, this study presents the model study for the alteration in solubility of drugs due to strong interactions between the component surfactants of mixed micellar systems, co-solubilization of differently architectured drugs and the interaction between the drugs in the mixed micellar media, being important both from the industrial as well as from the research point of view.
Experimental section
Materials
Tween20 (T20) was a Merck (India) product (purity > 99%) and Tween40 (T40) was obtained from Himedia laboratories (India) (purity > 99%). Brij30 (B30), Brij35 (B35), Brij56 (B56), Brij58 (B58) and cholic acid, sodium salt hydrate (NaC) amphiphiles were Aldrich products (purity > 99%). CBZ and NFD were Himedia laboratories (India) products (purity > 98%). All the chemicals were used as received. The chemical structures of the materials used are presented in Scheme 1.
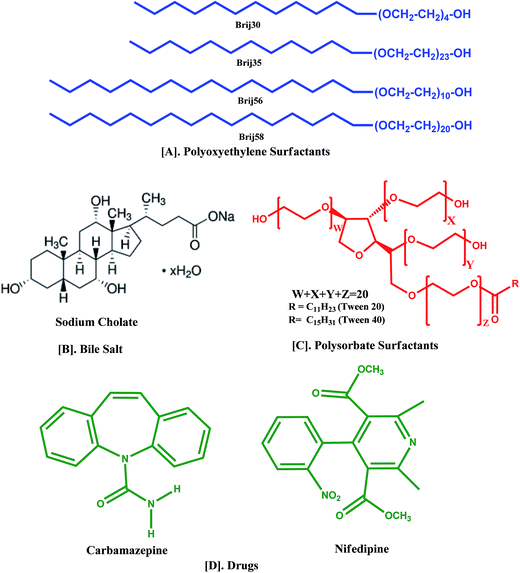 |
| | Scheme 1 Chemical structures of materials. | |
Methods
CMC determination. The cmc values were determined from the surface tension (γ) vs. log[surfactant] plotted in Fig. 1. Kruss 9 tensiometer was used to measure the surface tension by the platinum ring detachment method, having an accuracy of ±0.1 dyne cm−1. Surfactant concentration was varied by adding concentrated surfactant solution in small installments and readings were taken after a thorough mixing and temperature equilibration. The temperature was maintained at 25 °C (within ±0.1 °C) by circulating water from a HAAKE GH thermostat. The experiments were done in triplicate and the cmc values are presented as the mean of such measurements.
 |
| | Fig. 1 Variation of surface tension with surfactant concentration at 25 °C (a) C12 series (b) C16 series. | |
Fluorescence measurements. The aggregation numbers of pure and mixed surfactant systems were determined by steady-state fluorescence quenching experiments at 25 ± 0.1 °C. Pyrene was used as a probe with cetylpyridinium chloride (CPC) as quencher. The fluorescence emission spectra of pyrene were obtained with a Shimadzu, Japan, Model RF-5301 spectrofluorometer at excitation wavelength 336 nm and emission wavelength 373 nm. Measurements were made in a 1 cm path length quartz cuvette using a 3 mm excitation and emission slit width, and the fluorescence emission spectra were recorded in the range of 350–450 nm
Solubility experiment. The solubility and co-solubility of CBZ and NFD were measured in a range of in a range of 1–5 mM of total surfactant concentration. Excess amounts of drugs were added to the vials containing 3 ml of micellar solutions to ensure maximum solubility. The 5 ml sample vials were sealed with screw caps and agitated for a period of 24 hours with a magnetic stirrer at a temperature of (25 ± 0.5) °C using magnetic Teflon pieces previously placed in the vials. The solutions were subjected to centrifugation at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 rpm to remove the undissolved drug. The concentration of solubilized drug was determined spectrophotometricaly with a Shimadzu spectrophotometer (model UV-1650) following appropriate dilution of an aliquot of the supernatant with the corresponding surfactant concentration. The surfactant concentration was kept the same in both the reference and the measurement cells to eliminate the effect of the surfactant on the UV absorbance. The solubility of CBZ was determined at its characteristic wavelength of 286 nm, at which its extinction coefficient calculated from the calibration curve of the drug in methanol was 1.4335 mM−1 cm−1. Using this extinction coefficient, the solubility of CBZ in water was confirmed to be 6.98 × 10−1 mM, which tallied well with the previously reported value.40 The solubility of NFD was determined at 355 nm and was equal to 3.3 × 10−3 mM in conformity with earlier studies41 using extinction coefficient of 3.28 mM−1 cm−1, determined from the calibration curve of the drug in methanol. The solubilities of CBZ and NFD in the mixture were determined at the above mentioned wavelengths using their respective extinction coefficients. CBZ and NFD absorptions at 286 nm and 355 nm respectively were non-interfering with each other, as depicted by their prototype absorption curves in methanol (Fig. 2a) and in 2 mM (B35 + T20) binary surfactant solution (Fig. 2b). The concentrations of drugs are presented as the mean of three independent measurements corresponding to each surfactant concentration.
400 rpm to remove the undissolved drug. The concentration of solubilized drug was determined spectrophotometricaly with a Shimadzu spectrophotometer (model UV-1650) following appropriate dilution of an aliquot of the supernatant with the corresponding surfactant concentration. The surfactant concentration was kept the same in both the reference and the measurement cells to eliminate the effect of the surfactant on the UV absorbance. The solubility of CBZ was determined at its characteristic wavelength of 286 nm, at which its extinction coefficient calculated from the calibration curve of the drug in methanol was 1.4335 mM−1 cm−1. Using this extinction coefficient, the solubility of CBZ in water was confirmed to be 6.98 × 10−1 mM, which tallied well with the previously reported value.40 The solubility of NFD was determined at 355 nm and was equal to 3.3 × 10−3 mM in conformity with earlier studies41 using extinction coefficient of 3.28 mM−1 cm−1, determined from the calibration curve of the drug in methanol. The solubilities of CBZ and NFD in the mixture were determined at the above mentioned wavelengths using their respective extinction coefficients. CBZ and NFD absorptions at 286 nm and 355 nm respectively were non-interfering with each other, as depicted by their prototype absorption curves in methanol (Fig. 2a) and in 2 mM (B35 + T20) binary surfactant solution (Fig. 2b). The concentrations of drugs are presented as the mean of three independent measurements corresponding to each surfactant concentration.
 |
| | Fig. 2 Absorbance vs. wavelength of 1 mM (a) CBZ and NFD in methanol, and (b) CBZ, NFD and CBZ + NFD mixture in 2 mM B35 + T20 solution. | |
HPLC of CBZ and NFD during solubilization and co-solubilization. The liquid chromatography system consisted of a Shimadzu LC-20A with a SPD-M20A variable-wavelength UV detector (set at 237 nm), a CBM-20A/20Alite system controller, LC-20AB pump and an injection valve with a 25 μl loop (Shimadzu, Kyoto, Japan). Separation was achieved using Enable C18G column (250 mm × 4.6 mm, 5 μm) and CTO-10ASvp column oven. The mobile phase used consisted of water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (40
methanol (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60, v/v), flowing at a rate of 0.5 ml min−1. The instrument was operated at 40 °C. Drugs solubilized in mixed micellar solutions were first centrifuged and then filtered using a 0.2 μm filter paper. 20 μl of the drug solution was injected and the separation was carried out for 45 minutes.
60, v/v), flowing at a rate of 0.5 ml min−1. The instrument was operated at 40 °C. Drugs solubilized in mixed micellar solutions were first centrifuged and then filtered using a 0.2 μm filter paper. 20 μl of the drug solution was injected and the separation was carried out for 45 minutes.
Results and discussion
Surfactant–surfactant interactions in mixed micelles
The cmc values of NaC and mixed micellar systems for both series of surfactants involving 12 carbon (C12) and 16 carbon (C16) hydrophobic chain lengths are given in Table 1. The cmc values of T20, B30, B35, T40, B56 and B58 are 0.036, 0.033, 0.039, 0.029, 0.036 and 0.003 mM, respectively, as reported earlier by us.29 The experimental cmc values and cmcideal for mixed surfactant systems, calculated using Clint equation,42 are also given in Table 1. All the observed cmc values were found to be lower than the cmcideal values, indicating a negative deviation from the ideal behavior of the mixed micelle formation. The estimate of negative deviation of experimental cmc values from the cmcideal and hence the non-ideality of mixed binary surfactant systems was made in light of Rubingh's equation,43 based on the regular solution theory. The interaction parameter, β, is an indicator of the degree of interaction between two surfactants in mixed micelles and accounts for the deviation from ideality, and a negative β value indicates an attractive interaction. The values of β along with micellar mole fraction, XMi, and the activity coefficients, fi, of the ith surfactant calculated using Rubingh treatment are presented in Table 1. For ionic–nonionic mixed surfactant systems, the electrostatic self-repulsion of ionics and weak steric self-repulsion of nonionics are reduced by dilution effects after mixing.39 Moreover, the ion–dipole interaction between the hydrophilic head groups of anionic and nonionic surfactants favors the micellization and results in synergism.44–47 The favorable possibility of hydrogen bonding in addition to the polar attractions of the hydrophilic head groups of these two surfactant systems and the strong hydrophobic interactions of their tail groups may account for the obtained degree of non-ideality, which is in conformity with some earlier studies on such types of mixed micellar systems.39 The absolute magnitude of β increases with decrease in chain length of nonionic surfactant, a fact attributed to the more favorable self-interaction in longer hydrocarbon chain T40, B58 and B56 surfactant systems and hence higher propensity to form micelles.44 The large negative value of β for B30 + NaC (β = −8.77) and B56 + NaC (β = −10.42) binary mixed micellar systems in their respective series is due to lesser steric hindrance between the two surfactant systems involved, which promotes ease of micellization. The value of β for B35 + NaC (β = −7.97) is almost equal to that for T20 + NaC (β = −8.08) binary system and also β for T40 + NaC (β = −8.9) binary system is close to that of B58 + NaC (β = −9.11) binary mixed micellar system due to the comparable steric effects of surfactants involved. Among Brij-Tween binary systems, the larger interaction between B35 + T20 (β = −2.67) and B58 + T40 (β = −2.67) system is attributed to more polar-polar interactions between a large number of ethoxyl and hydroxyl groups present when compared to the interaction of B30 + T20 (β = −0.59) and B56 + T40 (β = −1.36) binary surfactant systems. Moreover, anionic–nonionic mixed micelles are dominated by non-ionic surfactants, as indicated by XMi values (Table 1) in conformity with the results of other studies on different ionic + non-ionic mixed micellar systems.48,49
Table 1 Critical micelle concentration (cmcexp), ideal critical micelle concentration (cmcideal), micellar composition (XMi), interaction parameter (β) and activity coefficients (fi) of equimolar binary surfactant mixtures using Rubingh's method and equimolar ternary surfactant mixtures using Rubingh's Pseudobinary and Rubingh–Holland methods at 25 °C for both C12 and C16 surfactant seriesa
| System |
cmcexp (mM) |
cmcideal/cmcRH(mM) |
β |
XM1/XM2/XM3 |
f1/f2/f3 |
| Error limits of cmc, X1, β and f are ±5%, ±0.02, ±0.05 and ±0.02, respectively. |
| NaC |
9.8 |
|
|
|
|
| B30 + NaC |
0.030 |
0.065 |
−8.77 |
0.76/0.24/— |
0.60/0.01 |
| B35 + NaC |
0.039 |
0.078 |
−7.97 |
0.77/0.23/— |
0.65/0.01 |
| B30 + T20 |
0.030 |
0.034 |
−0.59 |
0.48/0.52/— |
0.87/0.85 |
| B35 + T20 |
0.019 |
0.038 |
−2.67 |
0.51/0.49/— |
0.50/0.53 |
| T20 + NaC |
0.036 |
0.072 |
−8.08 |
0.77/0.23/— |
0.65/0.01 |
| B56 + NaC |
0.023 |
0.072 |
−10.42 |
0.72/0.28/— |
0.44/0.01 |
| B58 + NaC |
0.005 |
0.007 |
−9.11 |
0.76/0.25/— |
0.80/0.01 |
| B56 + T40 |
0.023 |
0.032 |
−1.36 |
0.53/0.47/— |
0.74/0.68 |
| B58 + T40 |
0.004 |
0.006 |
−2.67 |
0.73/0.27/— |
0.82/0.25 |
| T40 + NaC |
0.026 |
0.058 |
−8.90 |
0.75/0.25/— |
0.59/0.01 |
| B30 + T20 + NaC |
0.023 |
0.052/0.026 |
|
0.44/0.36/0.20 |
0.60/0.66/0.01 |
| B35 + T20 + NaC |
0.026 |
0.057/0.025 |
|
0.59/0.41/∼0.00 |
0.36/0.56/0.56 |
| B56 + T40 + NaC |
0.018 |
0.049/0.018 |
|
0.41/0.38/0.21 |
0.41/0.54/0.01 |
| B58 + T40 + NaC |
0.005 |
0.009/0.005 |
|
0.65/0.23/0.12 |
0.71/0.23/0.01 |
Holland and Rubingh have proposed a generalized multi-component non-ideal mixed micelle model based on a pseudophase separation approach. It has been successfully applied in case of many ternary surfactant systems50 for the evaluation of micellar composition, activity coefficients and cmc values. It makes an effective use of net interaction parameters determined experimentally from cmc measurements on binary systems. In the present study, values of binary interaction parameters β12, β13 and β23 calculated using Rubingh's method and cmc values of pure surfactants were used in the equations of Rubingh–Holland (RH) formulation50 to evaluate activity coefficients, f1, f2 and f3 and the micellar mole fractions XM1, XM2 and XM3. These values were then used to predict the cmc of ternary systems, cmcRH, according to Rubingh–Holland (RH) formulation. The results are presented in Table 1. The composition of mixed micelles (X) differs from the bulk composition (α), and Xanionic values are considerably lower than αanionic but fairly higher than αnonionic values in both surfactant series. The activity coefficients of anionics are very low but are close to unity for nonionics. The cmcRH values are found to be in good agreement with experimental cmc values, but both are lower than the cmcideal values, indicating the synergistic non-ideal nature of mixed ternary micellar systems. The agreement between cmcRH and experimental cmc in both the series indicates a fair applicability of the RH method for such systems.
Aggregation number
The mean aggregation number of pure and various binary and ternary surfactant systems were determined from steady state fluorescence data51 using the equation| |
 | (1) |
where, [Q], Ct and cmc are quencher concentration, total surfactant concentration and critical micelle concentration of the pure/mixed surfactant systems, respectively. I0 and I are the fluorescence intensities in the absence and presence of quencher, respectively, for the first vibronic peak in the pyrene emission spectra. A representative plot of decrease in fluorescence intensity of pyrene by the addition of CPC and that of ln(I0/I) versus [Q] for pure and equimolar binary and ternary mixed micellar systems are shown in Fig. 3a and b, respectively. The total surfactant concentration was kept constant at 10 mM, and the values of N (aggregation number) obtained for NaC, T20, and B30 were 12, 79 and 97, comparable to the earlier reported values29,39 of 11, 86 and 101, respectively, in aqueous. The aggregation number increases for binary and ternary micellar systems, indicating micellar growth. A correlation is observed between the aggregation number and the interaction parameter, where a high interaction corresponds to favorable micellization resulting in the formation of larger micelles. Bile salt micelles, due to the steric hindrance of the large steroidal skeleton, are small and have a low aggregation number. The addition of Brij/Tween surfactants favors the intermolecular hydrogen bonding between carboxyl, hydroxyl and ethoxy groups with partial hydrophobic interactions resulting in micellar growth, as reported in earlier studies.39 The most favorable interactions (β = −8.77) lead to a higher aggregation number of 167 for B30 + NaC binary micellar system. The aggregation number of T20 + NaC is 157 due to slightly less favourable interactions of the surfactants (β = −7.97) in mixed micelles compared to B30 + NaC binary surfactant system. The magnitude of interaction is even lesser for T20 and B30 surfactant systems, leading to a lower aggregation number (N = 127) than that of the other two systems. The aggregation number of the ternary surfactant system (T20 + B30 + NaC) is 131, which is more than that of the corresponding single surfactant systems but less or equal to that of their binary surfactant systems indicating a balance between electrostatic and steric effects.
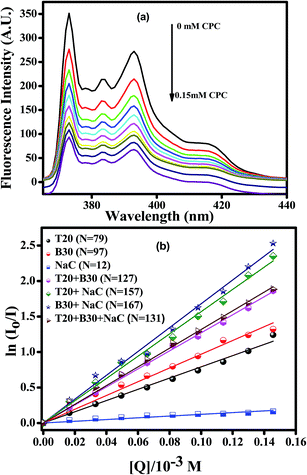 |
| | Fig. 3 Representative plot of (a) decrease in fluorescence intensity of pyrene with an increase in [CPC] (b) ln(I0/I) vs. [quencher] for the determination of the aggregation numbers of T20, B30, NaC and their equimolar binary and ternary combinations at 25 °C. | |
Molar solubilization ratio (MSR)
The molar solubilization ratio (MSR) is equivalent to an increase in solubilizate concentration per unit increase in micellized surfactant concentration. It is a measure of the effectiveness of a surfactant in solubilizing a given solubilizate. MSR is given by the equation44| |
 | (2) |
It is obtained from the slope of the curve between the solubilizate concentration and surfactant concentration. St is the total apparent solubility of a drug (CBZ or NFD) in either single state or in their binary mixture at a particular total single and/or mixed surfactant concentration, Ct, above cmc, and Scmc is the apparent solubility of drugs at cmc, which is taken as their water solubility because it changes very slightly up to the cmc of surfactant. The aqueous solubilities of drugs increase linearly over the range of single and/or mixed surfactant concentrations above cmc, indicating a solubility enhancement in water due to solubilization within the micelles. As a prototype, variation of St for CBZ in its single and mixed states vs. Ct in given mixed surfactant systems is shown in Fig. 4. The MSR values of CBZ and NFD individually as well as in combined states in the studied mixed surfactant systems calculated using the above mentioned procedure are presented in Table 2.
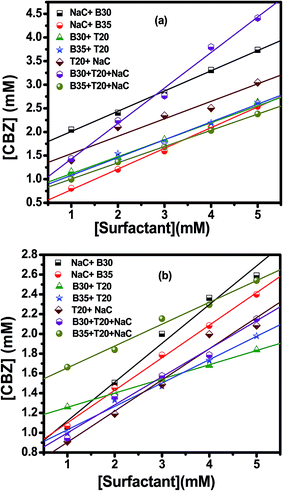 |
| | Fig. 4 Variation of the CBZ concentration with total surfactant concentration at 25 °C during (a) solubilization (b) co-solubilization. | |
Table 2 Experimental molar solubilization ratio (MSR) and ideal molar solubilization ratio (MSRideal) calculated for CBZ and NFD during solubilization and co-solubilization in various mixed surfactant systems at 25 °Ca
| Surfactant system |
CBZpure |
CBZmix |
NFDpure |
NFDmix |
| MSRideal |
MSR |
MSRideal |
MSR |
MSRideal |
MSR |
MSRideal |
MSR |
| Error limits in the measurement of MSR are ±6%. |
| B30 + NaC |
0.621 |
0.428 |
0.260 |
0.249 |
0.032 |
0.029 |
0.094 |
0.092 |
| B35 + NaC |
0.574 |
0.348 |
0.364 |
0.329 |
0.039 |
0.024 |
0.045 |
0.122 |
| B30 + T20 |
0.431 |
0.424 |
0.204 |
0.194 |
0.032 |
0.057 |
0.068 |
0.092 |
| B35 + T20 |
0.392 |
0.376 |
0.274 |
0.236 |
0.037 |
0.060 |
0.035 |
0.130 |
| T20 + NaC |
0.351 |
0.369 |
0.310 |
0.315 |
0.037 |
0.024 |
0.044 |
0.154 |
| B56 + NaC |
0.408 |
0.496 |
0.331 |
0.284 |
0.067 |
0.035 |
0.096 |
0.100 |
| B58 + NaC |
0.344 |
0.411 |
0.313 |
0.221 |
0.094 |
0.042 |
0.091 |
0.115 |
| B56 + T40 |
0.263 |
0.308 |
0.234 |
0.200 |
0.085 |
0.070 |
0.089 |
0.076 |
| B58 + T40 |
0.266 |
0.317 |
0.256 |
0.196 |
0.100 |
0.084 |
0.089 |
0.077 |
| NaC + T40 |
0.343 |
0.427 |
0.300 |
0.214 |
0.078 |
0.053 |
0.076 |
0.153 |
| B30 + T20 + NaC |
0.489 |
0.351 |
0.268 |
0.154 |
0.034 |
0.034 |
0.071 |
0.076 |
| B35 + T20 + NaC |
0.421 |
0.343 |
0.282 |
0.266 |
0.038 |
0.013 |
0.035 |
0.076 |
| B56 + T40 + NaC |
0.358 |
0.527 |
0.301 |
0.214 |
0.074 |
0.038 |
0.088 |
0.130 |
| B58 + T40 + NaC |
0.366 |
0.308 |
0.326 |
0.165 |
0.088 |
0.058 |
0.088 |
0.153 |
For NaC micelles, there occurs a slight increase in the solubilizate concentration by increasing the surfactant concentration below the cmc [Fig. 5a] due to weak interactions between the solubilizate monomer and the bile salt monomer, in accordance with earlier studies.52,53 The formation of micelles causes the solubilizate concentration to increase rapidly above the cmc [Fig. 5a]. Bile salt micelles provide nonpolar hydrophobic interior and polar hydrophilic surface for solubilization of polar steroids;54 therefore, in the present study, the amphiphilic drugs CBZ and NFD also seem to occupy both solubilization sites. The MSR plots of CBZ and NFD during solubilization and co-solubilization in NaC micelles are shown in Fig. 5b. NaC proves out to be a more promising medium for the solubilization of CBZ than all the other non-ionic surfactant systems studied29 due to more provision for the solubilization of CBZ in the outer hydrophilic corona as a result of favorable electrostatic interactions attributed to the presence of charge on the micellar surface. For NFD, the MSR values obtained in NaC are less than that of CBZ, and this difference in the solubility of two drugs could be attributed to the difference in their chemical structures. The incorporation of NFD into NaC micelles of relatively small aggregation number may need considerable energy to make a large space inside the micelle owing to its non-planer structure, higher molecular weight and higher molecular volume, resulting in its lesser solubilization within the micelle. The MSR value of NFD obtained in NaC is more than the MSR values obtained in micelles of the C12 series of non-ionic surfactants,29 which could be attributed to a higher palisade layer solubilization of the more polarizable NFD due to favorable polar interactions between the charged micelle-water interface of NaC and polar groups of NFD. However, the MSR value of NFD in NaC is lesser than its MSR value in C16 series of non-ionic surfactants, probably due to lesser aggregation number of NaC. This decreases micellar core volume available for NFD solubilization, which outplays the higher magnitude of solubilization at hydrophilic surface of NaC. During the co-solubilization of the two drugs in NaC, the solubility of CBZ is decreased, probably due to the preferential occupation of NaC micellar core by NFD. CBZ solubilized in the palisade layer of NaC micelle decreases the micelle-water interfacial tension, and hence, it increases the micellar core volume, thereby increasing the solubilization of NFD.
 |
| | Fig. 5 (a) Plot showing an increase in solubility of drugs along with an increase in the NaC concentration in pre and post micellar regions. (b) Variation of concentration of CBZ and NFD during solubilization and co-solubilization with NaC surfactant concentration. | |
CBZ is an amphiphilic drug molecule, whereas NFD is slightly polar but polarizable drug molecule. Therefore, these drugs can be solubilized both in the core and in the palisade layer of the micelle.29 The surfactant systems that exhibit synergism on mixing not only show decrease in the cmc values but also an increase in the aggregation number. In strongly interacting surfactant systems, the aggregation number is higher (as observed in mixed systems of B30, T20 and NaC), indicating micellar growth compared to their single surfactant micelles.55,57 The change is sensitive to those solutes that solubilize by incorporation in the micellar hydrocarbon core. The application of Laplace pressure to the mixed micelle situation predicts an increase of the micellar solubilization upon micellar growth. Furthermore, the electrostatic attraction between polar head groups of surfactants in the mixed micellar system reduces the interaction between surfactants and amphiphilic solubilizates.55–57 The larger increase of the aggregation number in the mixed micelle results in enhanced solubilization in micellar core, whereas the strong interaction between the surfactant head groups lowers the solubilization in the palisade layer. Therefore, the occurrence of synergism in solubilization of substances in mixed micelles depends on the relative strength of the two opposing effects. When a larger part of the solubilizate is located in the micellar core, a positive synergism is expected. In contrast, if the palisade layer solubilization exceeds, then the solubilization capacity of mixed micelle is smaller than that of pure micelles.
CBZ solubilized in C12 series of surfactants shows a decrease in MSR values when compared to ideal MSR values (MSRideal), calculated as
| | |
MSRideal = X1MSR1 + X2MSR2
| (3) |
where
X1 and
X2 are mole fractions of the two surfactants and MSR
1 and MSR
2 are the molar solubilization ratios for CBZ
29 in the two surfactant solutions involved. The MSR
ideal values of CBZ and NFD calculated using
eqn (2) are given in
Table 2. Because an appreciable amount of CBZ is solubilized in the palisade layer,
29 the decrease in MSR values could be attributed to the decrease in polar interaction between the surfactants and CBZ due to mixing effect of surfactants. The results are in agreement with the solubilization of polar hydrophobic molecules, such as barbituric acid,
55 n-hexanol,
56 1-petanol
57 and
n-octanol,
58 solubilized in the mixed micelles with larger negative
β values. The decrease in solubilization of CBZ occurs considerably more for the B35 + NaC binary surfactant system owing to the larger interaction between the surfactants involved, as indicated by larger negative values of
β. Moreover, the main solubilization site of CBZ in B35 is the palisade layer,
29 where polar interactions between the amide group of CBZ and OE groups of B35 determine its solubility. The mole fraction of B35 in mixed micelle is more as calculated by Rubingh method [
Table 1]; therefore, the decrease in these interactions due to the mixing effect of surfactants decreases the MSR value of CBZ. The difference between the experimental MSR and MSR
ideal is the least for the B30 + T20 surfactant systems, attributed to the least negative value of
β, and because the surfactant mixing approaches an ideal behavior, the solubilization behavior also approaches ideality. Due to the larger core volume of C
16 series of surfactants, CBZ is mainly solubilized in the micellar core;
29 therefore, a further increase in the aggregation number, and hence in its micellar core volume, increases its solubility. The MSR values of CBZ obtained in C
16 series of surfactants are more than the ideal values due to the higher aggregation number upon surfactant mixing, leading to favourable solubilization in the hydrocarbon core, as predicted by the basic Laplace pressure equation. In the case of toluene, which is a slightly polar but polarizable molecule,
59 as well as in other slightly soluble benzenoid compounds.
60 there is a similar behavior in surfactant systems with longer chain lengths where the increase in solubilization occurs due to an increase of aggregation number in the mixed micellar system. In the C
16 series, the interaction parameter has a larger negative value for the B56 + NaC binary surfactant system; therefore, the increase in micellar core volume will be maximum, and hence the solubility of CBZ is large. As mentioned earlier, the B56 + NaC binary mixture is dominated by B56, and as a result we expect a higher core volume for such a binary system. Moreover, the main solubilization site for CBZ in B56 + NaC binary mixture being micellar core makes the higher core volume to favor the solubilization of CBZ, explaining the bigger difference in the magnitude of experimental MSR than MSR
ideal for this binary surfactant system. The difference between the experimental MSR value and the MSR
ideal is least for the B56 + T40 binary surfactant system, corresponding to the lowest interaction between the surfactant systems involved. The difference between the MSR
ideal and the experimental MSR values follows the trend of
β values; it is less for the systems with lower negative values of
β and more for the systems with more negative
β values. In the ternary mixed micellar systems, a negative synergism in solubilization is observed for B30 + T20 + NaC, B35 + T20 + NaC and B58 + T40 + NaC surfactant systems, probably due to the more favorable interactions between the surfactants involved, which decreases the palisade layer solubilization and hence the total solubility of CBZ in these systems. For the B56 + T40 + NaC mixed micellar system, the increase in the aggregation number and the micellar core solubilization seems to override the effect of the decrease in the palisade layer solubilization. Synergism in solubilization greatly depends on the molecular structure and polarity of solubilizates.
59–61 For NFD there occurs a decrease in solubility when compared with the ideal solubilization in both the surfactant series due to its higher polarizability, and hence in its appreciable solubilization at the palisade layer.
29 However, for the B30 + T20 and B35 + T20 binary surfactant systems, there occurs an increase in solubility of NFD due to the relatively higher increase in the micellar core solubilization compared to the decrease in the palisade layer solubilization, as the surfactants involved have lesser interactions, as indicated by the lower negative value of
β. The difference between the experimental MSR and MSR
ideal values is higher for the surfactant systems with more interactions, as the probability for hydrophilic interactions between the drugs and surfactants of mixed micelle decreases with a decrease in the magnitude of
β. The decrease in solubility of NFD is higher for the B35 + NaC and B58 + NaC surfactant systems than its decrease in the B30 + NaC and B56 + NaC binary surfactant systems, respectively, due to a more appreciable palisade layer solubilization of NFD in B35 and B58 surfactant systems, and hence, there is more provision of decrease in its solubility due to the strong interactions between the surfactants in binary mixed micellar systems. For NFD solubilized in ternary surfactant systems, an ideal solubilization of NFD is obtained in the B30 + T20 + NaC mixed micellar system, where the increase in core volume and decrease in palisade layer solubilization seem to balance each other. For the rest of the ternary mixed micellar systems, negative synergism in solubilization is observed due to less solubilization in dense hydrophilic corona associated with these surfactant systems, where the provision for solubilization is less due to surfactant–surfactant interactions and steric effects.
Co-solubilization of CBZ and NFD
MSR values of CBZ and NFD during their co-solubilization are also presented in Table 2. It is observed from the data that MSR values of CBZ during its co-solubilization are reduced in all surfactant systems relative to its MSR values during single solute solubilization. It has been suggested that the location of solutes within micelles is an important factor influencing the micellar partitioning of solutes in multi-solute systems.62–64 If the solutes compete with each other for a location in the interior of micelle, it will lead to a decrease in the solubility of one solute in the presence of others. However, during the co-solubilization of two solutes with different hydrophobicities, the less hydrophobic compound gets solubilized in the palisade layer of the micelle, resulting in an increase in the micellar core volume that allows a synergistic increase in the MSR value of the more hydrophobic compound, which prefers the micellar core solubilization.62–64 CBZ, being more polar, is appreciably solubilized at palisade layer, whereas its solubilization within the micellar core is decreased during co-solubilization due to the preferential occupation of the micellar core by more hydrophobic NFD, resulting in a net decrease in CBZ solubility. The experimental MSR values of CBZ during co-solubilization are less when compared with the MSRideal values calculated for co-solubilization. The deviation from the ideal solubility is more during co-solubilization because of the lower potency of the palisade layer towards the solubilizates in mixed micellar media due to the favorable polar interactions between the component surfactants of mixed micelles. Because during co-solubilization the palisade layer is the main occupation site of CBZ,29 its solubility is affected the most. The solubility decreases along with the increase in interactions between the surfactants involved; it is more for the B35 + NaC and B58 + NaC surfactant systems, which show more synergism in mixed micellization, and have higher mole fractions of B35 and B58 in the mixed micelle, and therefore, they offer more palisade layer solubilization. The decrease in solubility is least for the B30 + T20 and B56 + T40 binary surfactant systems, exhibiting the minimum synergism, as indicated by the lower negative value of β and the less potency of B30 and B56 towards palisade layer solubilization. In the case of ternary surfactant systems, the MSR value of CBZ obtained during co-solubilization is lower than the MSR value of CBZ obtained for single solute solubilization due to the competitive solubilization behavior exhibited by the two drugs. The decrease is higher in the B30 + T20 + NaC and B56 + T40 + NaC ternary micellar systems due to the higher mole fractions of Brij30 and Brij56, respectively (Table 1). Therefore, there is more provision for micellar core solubilization, which is occupied by NFD during co-solubilization. Comparison between experimental MSR values and MSRideal values (calculated using eqn (2)) reveals a decrease in CBZ solubility in all ternary surfactant systems due to the preferential occupation of the palisade layer by CBZ during co-solubilization. A further decrease is observed in the ternary mixed micellar system of B35 and B58 due to more interactions between the surfactants involved. Moreover, higher cmc, and hence lower palisade layer area than the individual surfactants, also decreases the solubility of CBZ.
There occurs an increase in the MSR value of NFD during co-solubilization that is attributed to an increase in the micellar core volume by the solubilization of the less hydrophobic CBZ in the palisade layer of the micelles, which decreases the interfacial tension. CBZ would be replaced from the core of the micelle by the more hydrophobic NFD during its co-solubilization in the two solutes system, which on one hand, results in a drastic decrease in the MSR value of CBZ, and on the other hand, CBZ solubilized in the palisade layer makes the stay of NFD in the micellar core more favorable. In general, the experimental MSR values obtained for NFD in all the surfactant systems are higher than the MSRideal values obtained using eqn (2), except for the B30 + NaC, B56 + T40 and B58 + T40 binary surfactant systems, where the experimental MSR values are slightly lower than the MSRideal values, probably due to a decrease in the palisade layer solubilization and more competition with the CBZ for the micellar core solubilization. The enhanced solubility of NFD compared to ideal solubilization in all other mixed micellar systems during co-solubilization can be attributed to two phenomena, which cause the micellar core volume to increase, thereby increasing the solubilization of NFD.
1. Formation of bigger micelles: Due to the favorable interactions between the surfactants involved in the mixed micellar systems, the propensity of micellization increases. Bigger micelles with larger core volumes are formed, and hence during co-solubilization an enhancement in solubilization of NFD is observed compared to the solubilization of NFD in single surfactant systems.29
2. Decreased interfacial tension: CBZ solubilized in the palisade layer decreases the interfacial tension at micelle–water interface, thereby increasing the micellar core volume, and hence the solubility of NFD.Although the two phenomena lead to the same result, a strong interaction between the surfactants involved in mixed micelle decreases the palisade layer solubilization of CBZ. Therefore, an overall effect of the two phenomena seems to be operative where a stronger interaction between the surfactant systems and a higher magnitude of CBZ solubilized in the palisade layer in a given micelle favors the solubilization of NFD. This explains the larger difference between the experimental MSR values and MSRideal values in the T20 + NaC, B35 + NaC and T40 + NaC surfactant systems when compared to the rest of the binary surfactant systems. For the ternary surfactant systems, an enhancement in solubility of NFD is observed during co-solubilization, and also the experimental MSR values are higher than the MSRideal values, which is more prominent in the ternary surfactant systems involving B35 and B58 due to an appreciable amount of CBZ solubilized at the micelle-water interface.
HPLC of CBZ and NFD during solubilization and co-solubilization
The HPLC profiles of CBZ and NFD during solubilization and co-solubilization in binary (T20 + NaC) and ternary (B30 + T20 + NaC) surfactant system are given in Fig. 6. It is pertinent to mention that the surfactants did not elute at the time range presented in the HPLC profiles. The molar absorbance coefficient being different for the two drugs, the intensity of the peaks cannot be directly correlated with their concentration. When the relative concentration of drugs during a single solute solubilization and co-solubilization is compared, the intensity of the peaks corresponding to both drugs decreases during the co-solubilization, indicating competition between the drugs for solubilization sites. Furthermore, the relative amount of drugs solubilized in the (T20 + NaC) binary surfactant system is more than that of the (B30 + T20 + NaC) ternary surfactant system, which is in agreement with the results presented in Table 2. During co-solubilization, the retention time of NFD was increased from 1.51 to 2.9 minutes and 1.03 to 2.7 minutes, whereas that of CBZ was slightly decreased from 3.3 to 3.14 minutes and 3.4 to 3.25 minutes in the binary and ternary surfactant mixture, respectively, which confirms that the drugs show interaction within the mixed micellar systems. The magnitude of interaction is lesser in the ternary surfactant system compared to the binary micellar system, where the two drugs have a tendency to get eluted together (Fig. 6c), as per the results presented in Table 3.
 |
| | Fig. 6 HPLC profile (a) NFD (b) CBZ (c) NFD + CBZ in (T20 + NaC) binary surfactant system (d) NFD (e) CBZ and (f) CBZ + NFD in (B30 + T20 + NaC) in ternary surfactant system. | |
Table 3 Excess Gibbs energy changes (ΔGSexcess), interaction parameter (ω/RT) and activity coefficients (γi) of CBZ and NFD during co-solubilization in different mixed surfactant systems at 25 °Ca
| System |
ΔGSexcess |
ω/RT |
γCBZ |
γNFD |
| ΔGSexcess is in kJ mol−1 with error limits ±4%. |
| B30 + NaC |
−3.69 |
−0.0076 |
0.999 |
0.996 |
| B35 + NaC |
−5.10 |
−0.0104 |
0.999 |
0.995 |
| B30 + T20 |
−3.07 |
−0.0057 |
0.999 |
0.997 |
| B35 + T20 |
−4.36 |
−0.0077 |
0.999 |
0.997 |
| T20 + NaC |
−5.77 |
−0.0106 |
0.999 |
0.995 |
| B56 + NaC |
−3.71 |
−0.0078 |
0.999 |
0.996 |
| B58 + NaC |
−4.12 |
−0.0074 |
0.999 |
0.997 |
| B56 + T40 |
−2.82 |
−0.0057 |
1.000 |
0.997 |
| B58 + T40 |
−2.65 |
−0.0053 |
1.000 |
0.997 |
| NaC + T40 |
−4.74 |
−0.0079 |
0.999 |
0.997 |
| B30 + T20 + NaC |
−2.11 |
−0.0038 |
1.000 |
0.998 |
| B35 + T20 + NaC |
−3.85 |
−0.0090 |
1.000 |
0.995 |
| B56 + T40 + NaC |
−4.34 |
−0.0075 |
0.999 |
0.997 |
| B58 + T40 + NaC |
−4.77 |
−0.0077 |
0.998 |
0.998 |
Partition coefficient
The effectiveness of solubilization can also be expressed in terms of the partition coefficient, Km, of the drug between the micelle and aqueous phases, and is defined as the ratio of mole fraction of the drugs in the micellar phase, Xm, to that in the aqueous phase, Xa.| |
 | (4a) |
The value of Xm in terms of MSR can be written as
| |
 | (4b) |
During co-solubilization of two species ‘i’ and ‘j’, the mole fraction of the drug ‘i’, Xmi, is given by53
| |
 | (4c) |
where
ni,
nj and
nsurf are the number of moles of solutes (
i and
j) and surfactant in micellar pseudo-phase, respectively.
Xa can be expressed as
Xa = [
Scmc]
Vm.
Vm is the molar volume of water equal to 0.01805 L mol
−1 at 25 °C. With these expressions, the
Km for solubilization becomes
| |
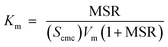 | (5a) |
For the co-solubilization partition coefficient, Kmi, of the drug i would be given by
| |
 | (5b) |
The Km and Kmi values of the two drugs are given in Fig. 7 and 8 for all mixed micellar systems.
 |
| | Fig. 7 Comparison of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Km and log Km and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K(x) of CBZ during single solute solubilization and co-solubilization in (a) C12 series (b) C16 series. K(x) of CBZ during single solute solubilization and co-solubilization in (a) C12 series (b) C16 series. | |
 |
| | Fig. 8 Comparison of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Km and log Km and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K(x) of NFD during single solute solubilization and co-solubilization in (a) C12 series (b) C16 series. K(x) of NFD during single solute solubilization and co-solubilization in (a) C12 series (b) C16 series. | |
According to Treiner et al.65 the partitioning of a neutral nonpolar solutes between a pseudo binary micellar solution and water is represented by the relationship
| |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K(x) = xm K(x) = xm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K1 + (1 − xm)ln K1 + (1 − xm)ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K2 + xm(1 − xm)B K2 + xm(1 − xm)B
| (6) |
where
K1 and
K2 are mole fractional partition coefficients of the solute in the single surfactant solutions and
K(
x) is the same parameter in the mixed micelles.
B66 is an empirical parameter and is given by
where
β is the interaction parameter appearing in the regular solution model for the cmc of surfactant mixtures in the absence of any solubilizate.
For the solubilization of CBZ, the K(x) values calculated using eqn (6) are smaller than the partition coefficient for the single surfactant micelles;29 therefore, there occurs a decrease in solubilization of CBZ due to strong interactions between the surfactants leading to a decreased palisade layer solubilization, both during its solubilization and co-solubilization in mixed micellar systems. The results are in conformity for other amphiphilic solubilizates in anionic-nonionic mixed micellar system having negative β values.67,68 K(x) values obtained were compared with the Km and Kmi values calculated using eqn (5a) and (5b) for solubilization and co-solubilization of CBZ (Fig. 7). A good correlation was attained, though K(x) values obtained were smaller than the Km and Kmi values, apparently due to the polar nature of CBZ as the eqn (6) has been developed for the partitioning of a neutral nonpolar solutes between a pseudo binary micellar solution and water.65 NFD seems to follow the ideal solubilization behavior with the K(x) values calculated using eqn (6) very close to the partition coefficient for the pure component micelle.29 The solubilization of pentanol69 in an anionic + non-ionic mixed micelle system is found to follow an ideal behavior, with β = 0 due to small aggregate structural changes. For NFD solubilization in mixed micelles, β ≠ 0 but the increase in solubilization due to larger aggregate structural changes is balanced by the decrease in solubilization in the palisade layer due to appreciable surfactant interactions. A good correlation was also observed between the K(x) values calculated for NFD using eqn (6) and the Km and Kmi values calculated using eqn (5a) and (5b) for its solubilization and co-solubilization (Fig. 8). For NFD, the K(x) values obtained were greater than the Km and Kmi attributed to its appreciable polarizability. The variation of ideal partition coefficients and the experimental partition coefficients could be attributed to the competition between the two opposite effects of increase in its solubilization in the micellar core and decrease in its solubility in the palisade layer, depending on the polarity and other physico-chemical properties of solubilizates.
Drug–drug interaction in the micellar pseudophase
Solubilized amounts of CBZ and NFD during solubilization and co-solubilization as well as total solubilized amounts of both drugs during co-solubilization for B35 + NaC system were plotted against the surfactant concentration, as shown in Fig. 9. The total solubilized amount of drugs (CBZ + NFD) solubilized during co-solubilization is greater than both the amount of CBZ and NFD solubilized during the single solute solubilizations for each surfactant system, indicating their synergistic solubilization during co-solubilization. To reveal the nature of interactions between drugs inside the micelles, the formulation proposed by Sugihara et al.70 has been adopted:
 |
| | Fig. 9 Comparison between solubilized amounts of CBZ and NFD during solubilization and co-solubilization in addition to total amount of the two drugs solubilized in B35 + NaC binary surfactant systems at 25 °C. | |
The solubilization equilibrium when a drug is used in excess can be written as
| |
 |
(11)
|
where,
Kd = activity of singly dispersed drug/activity of solid drug and is equal to the activity of the singly dispersed drug because the activity of the solid drug is unity. Since the drug solubility is very low,
Kd approximates to molarity of drug solubilized below cmc, which is equal to its water solubility,
Scmc. The equilibrium constant of solubilization for translation from a solid phase to a solubilized state in micelles is, therefore, given by
where
Km is the partition coefficient of the drug between aqueous phase and micellar phase. Values of
Km were calculated from
eqn (5a) for single solubilizate systems. The molar Gibbs free energy change upon solubilization, Δ
G°, is therefore given as
| |
ΔG° = − RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Keq Keq
| (9) |
For co-solubilization of two solid solubilizates, A and B, the total equilibrium constant of cosolubilization for translation from solid phase to solubilized state in micelles is given by
| | |
Kmixeq = (KAd × KAm) × (KBd × KBm)
| (10) |
where
KAd and
KBd are the respective activities of the two drugs A and B dispersed in bulk and
KAm and
KBm are their respective partition coefficients in mixed solubilization systems. Taking the
KAm and
KBm from
eqn (5b), the Gibbs energy change, Δ
G°
m, accompanying the translation of two solubilizates from bulk phase to micellar phase would be given by:
| |
ΔG°mix = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kmixeq Kmixeq
| (11) |
If the mixture is ideally formed, the molar Gibbs energy of ideal mixing ΔGsmix(ideal) should satisfy the additivity rule as
| | |
ΔGsmix(ideal) = χAΔG°A + χBΔG°B
| (12) |
where
χA and
χB are the mole fractions of the two species ‘A’ and ‘B’ within the micelles on the solubilizate only basis, and were calculated from the equation
| |
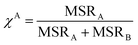 | (13) |
where the MSR
i were taken as their MSR values during co-solubilization. The difference between the real value of the free energy change Δ
G°mix and Δ
Gsmix(ideal) gives the excess Gibbs energy
| | |
ΔGsexcess = ΔG°mix − (χAΔG°A + χBΔG°B)
| (14) |
Because the total amount of two drugs solubilized during co-solubilization is more than the amount of CBZ and NFD solubilized during single solute solubilization, ΔGSexcess is negative for all the surfactant systems.
The interaction parameter ‘ω/RT’, activity coefficients of the two drugs inside the micelles ‘γc’ and ‘γn’ are calculated from the excess Gibbs energy70 ΔGSexcess as,
| | |
ω = ΔGSexcess/(χAχB)RT
| (15) |
| |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) γi = ω(1 − χA)2/RT γi = ω(1 − χA)2/RT
| (16) |
The values of ΔGSexcess, ω/RT and γi's calculated in different surfactant systems for the drugs are presented in Table 3. The interaction parameter ‘ω/RT’ gives the cohesive forces between the unlike solubilizates. The negative values of ‘ω/RT’ obtained signify that the interactions between CBZ and NFD were enhanced, and the two drugs are spontaneously miscible in all the mixed micellar systems studied. The absolute magnitude of ω/RT obtained in mixed micelles is higher than that of single surfactant systems,29 indicating that there is more favorable interactions between the two drugs in the mixed micellar media. The results obtained are quite evident, as the surfactants in the mixed micellar system firmly interact among themselves, and hence, their interaction with the drugs is less, which enforces the intermolecular interaction between the drugs. Moreover, due to the negative β value, the palisade layer solubilization of drugs is less in all the mixed micellar systems studied and there occurs an increase in the micellar core volume, and hence an appreciable and more sterically favored drug solubilization in the micellar core. The two drugs are in close proximity in the micellar core where there are more chances of favorable polar interactions between the two drugs within the nonpolar environment of the micellar core. The trend obtained for the interaction parameter ‘ω/RT’ is the same as that for β values. A larger negative value of β corresponds to more interactions between the surfactants, and hence a larger increase in micellar core volume, which results in more favorable interactions between the drugs. The interaction parameter ‘ω/RT’ is higher for the T20 + NaC, B35 + NaC and T40 + NaC binary mixed micellar systems attributed to a higher increment in the core volume and a weaker hydrophobicity within these mixed micelles due to their higher HLB values, both of which favor the interaction between the drugs. The interaction parameter ‘ω/RT’ has a lower value for the B30 + T20, B56 + T40 and B58 + T40 binary surfactant systems owing to the lower negative value of β, and a higher hydrophobicity within these mixed micellar systems. In the case of the ternary surfactant systems, the interaction between the drugs is higher in the B35 + T20 + NaC mixed micellar system because of its lower hydrophobicity due to the presence of the higher mole fraction of B35 in the mixed micelle, whereas the interaction between the drugs is less in B30 + T20 + NaC due to the higher hydrophobicity of B30, which is present in the higher mole fraction in the mixed micelles.
Conclusions
An estimation of the interaction between the surfactants in mixed micelles is important to understand the role of a number of amphiphiles in biological systems. In the present study, solubilization and co-solubilization of drugs in aqueous amphiphillic solutions containing Bile Salt surfactant micelles and its mixed micelles with non-ionic surfactants, viz Tweens and Brijs, were investigated. The solubilization of drugs depends on the interaction between the surfactants in mixed micelles, it increases with increase in interactions between the surfactants when the location of solubilization is the micellar core, and decreases with increase in increase in surfactant interactions when the solubilization occurs in the palisade layer. From experimental measurements of the surfactant–surfactant interaction during mixed micellization, the amount of drugs solubilized during a single solute solubilization and co-solubilization, the interaction between the drugs and the thermodynamic analysis provide a quantitative understanding of the solubilization of these drugs with mixed micellar systems. This work also emphasizes the importance of mixed surfactant systems that can be frequently used for transport and drug delivery purposes.
Acknowledgements
We are thankful to the Head, Department of Chemistry, University of Kashmir, for his constant encouragement and inspiration. AAD acknowledges Department of Science and Technology (DST), Govt of India for providing funds under the FIST (level-I) scheme to the Department of Chemistry, University of Kashmir for procuring various instruments.
References
- J. Bhattacharjee, G. Verma, V. K. Aswal, A. A. Date, M. S. Nagarsenker and P. A. Hassan, J. Phys. Chem. B, 2010, 114, 16414–16421 CrossRef CAS PubMed.
- C. Rupp, H. Steckel and B. W. Muller, Int. J. Pharm., 2010, 387, 120–128 CrossRef CAS PubMed.
- M. A. Hammad and B. W. Muller, Eur. J. Pharm. Biopharm., 1998, 46, 361–367 CrossRef CAS.
- L. Jiang, K. Wang, M. Deng, Y. Wang and J. Huang, Langmuir, 2008, 24, 4600–4606 CrossRef CAS PubMed.
- T. R. Bates, M. Gibaldi and J. L. Kanig, J. Pharm. Sci., 1966, 55, 191–199 CrossRef CAS.
- M. A. Kassem, A. G. Mattha, A. E. M. El-Nimr and S. M. Omar, Int. J. Pharm., 1982, 12, 1–9 CrossRef CAS.
- T. T. Kararli and V. W. Gupta, J. Pharm. Sci., 1992, 81, 483–485 CrossRef CAS.
- P. E. Luner, S. R. Babu and G. W. Radebaugh, Pharm. Res., 1994, 11, 1755–1760 CrossRef CAS.
- P. P. Nair and D. Kritchevsky, The Bile Acids: Chemistry, Physiology and Metabolism, Plenum Press, NY, 1971, vol. 1, pp. 1–9 Search PubMed.
- D. J. Parks, S. G. Blanchard, R. K. Bledsoe, G. Chandra, T. G. Consler, S. A. Kliewer, J. B. Stimmel, T. M. Willson, A. M. Zavacki, D. D. Moore and J. M. Lehmann, Science, 1999, 284, 1365–1368 CrossRef CAS.
- V. L. Sallee and J. M. Dietschy, J. Lipid Res., 1973, 14, 475–484 CAS.
- H. Westergaard and J. M. Dietschy, J. Clin. Invest., 1976, 58, 97–108 CrossRef CAS PubMed.
- F. A. Wilson, Am. J. Physiol., 1981, 241, 83–92 Search PubMed.
- J. R. O'Reilly, O. I. Corrigan and C. M. O'Driscoll, Int. J. Pharm., 1994, 109, 147–194 CrossRef.
- P. Garidel, A. Hildebrand and A. Blume, Molecules, 2007, 12, 2292–2326 CrossRef CAS.
- J. S. Dangi, S. P. Vyas and V. K. Dixit, Drug Dev. Ind. Pharm., 1995, 21, 2021–2027 CrossRef CAS.
- T. S. Wiedmann and L. Kamel, J. Pharm. Sci., 2002, 91, 1743–1764 CrossRef CAS PubMed.
- T. S. Wiedmann, W. Liang and L. Kamel, Pharm. Res., 2002, 19, 1203–1208 CrossRef CAS.
- M. A. Hammad and B. W. Müller, Eur. J. Pharm. Biopharm., 1998, 46, 361–367 CrossRef CAS.
- G. Dongowski, B. Fritzsch, J. Giessler, A. Härtl, O. Kuhlmann and R. H. H. Neubert, Eur. J. Pharm. Biopharm., 2005, 60, 147–151 CrossRef CAS PubMed.
- M. A. Hammad and B. W. Müller, Drug Dev. Ind. Pharm., 1999, 25, 409–417 CrossRef CAS PubMed.
- J. Guo, T. Wu, Q. Ping, Y. Chen, J. Shen and G. Jiang, Drug Delivery, 2005, 12, 35–39 CrossRef CAS.
- Handbook of Pharmaceutical Excipients, ed. A. H. Kibbe, Pharmaceutical Press, London, 3rd edn, 2000, pp. 407– 423 Search PubMed.
- N. Akhter, M. U. Rehman, H. M. S. Khan, F. Rasool and T. Saeed, Trop. J. Pharm. Res., 2011, 10, 281–288 Search PubMed.
- T. Cserhati, Environ. Health Perspect., 1995, 103, 358–364 CrossRef CAS.
- Y. Kapoor and A. Chauhan, Int. J. Pharm., 2008, 361, 222–229 CrossRef CAS PubMed.
- A. Acharya, S. K. Sanyal and S. P. Moulik, Curr. Sci., 2001, 4, 25 Search PubMed.
- S. Ajith and A. K. Rakshit, Langmuir, 1995, 11, 1122–1126 CrossRef CAS.
- M. Maswal, N. Islam, A. H. Pandith and A. A. Dar, J. Solution Chem., 2013, 42, 1374–1392 CrossRef CAS.
- L. S. Cheng, A. N. Prasad and M. J. Rieder, The Canadian Journal of Clinical Pharmacology, 2010, 17, 5–46 Search PubMed.
- G. A. Shehata, A.-A. Bateh, S. A. Hamed, T. A. Rageh and Y. B. Elsorogy, Neuropsychiatr. Dis. Treat., 2009, 5, 527–533 CrossRef CAS.
- N. E. Bharucha, Epilepsia, 2003, 44, 9–11 CrossRef.
- D. G. Mcdevitt, D. Currie, A. N. Nicholson, N. A. Wright and M. B. Zetlein, Br. J. Clin. Pharmacol., 1991, 32, 541–549 CrossRef CAS PubMed.
- R. G. Miller, Pharmacol. Rev., 1986, 38, 324–427 Search PubMed.
- L. K. Desmedt, C. J. Niemegeers and P. A. Tansson, Arzneim. Forsch., 1975, 25, 1408–1413 CAS.
- S. Joseph, J. David and T. Joseph, Indian J. Physiol. Pharmacol., 1998, 42, 383–388 CAS.
- R. I. Brahmane, V. V. Wanmali, S. S. Pathak and K. J. Salwe, J. Pharmacol. Pharmacother., 2010, 1, 78–81 CrossRef CAS PubMed.
- P. D. Garg, P. Singh and N. Kumar, Indian J. Psychiatry, 1998, 40, 389–391 CAS.
- M. Maswal and A. A. Dar, Colloids Surf., A, 2013, 436, 704–713 CrossRef CAS PubMed.
- W. Liu, L. Dang, S. Black and H. Wei, J. Chem. Eng. Data, 2008, 53, 2204–2206 CrossRef CAS.
- F. Sardari and A. Jouyban, Ind. Eng. Chem. Res., 2013, 52, 14353–14358 CrossRef CAS.
- J. H. Clint, J. Chem. Soc., Faraday Trans. 1, 1975, 71, 1327–1334 RSC Micellization of mixed non-ionic surface active agents.
- D. N. Rubingh, Mixed Micelle Solutions, in Solution Chemistry of Surfactants, ed. K. L. Mittal, Plenum Press, New York, 1979, pp. 337–354 Search PubMed.
- A. A. Dar, G. M. Rather and A. R. Das, J. Phys. Chem. B, 2007, 111, 3122–3132 CrossRef CAS PubMed.
- D. Kolp, R. Laughlin and R. Zimmerer, J. Phys. Chem., 1963, 67, 51 CrossRef CAS.
- C. M. Nguyen, J. F. Rathman and J. F. Scamehorn, J. Colloid Interface Sci., 1986, 112, 438 CrossRef CAS.
- M. J. Rosen and X. Y. Hua, J. Colloid Interface Sci., 1982, 86, 164 CrossRef CAS.
- F. J. Carrion, Tenside Deterg., 1985, 22, 5 Search PubMed.
- S. K. Mehta, S. Chaudhary, R. Kumar and K. K. Bhasin, J. Phys. Chem. B, 2009, 113, 7188–7193 CrossRef CAS PubMed.
- P. M. Holland and D. N. Rubingh, J. Phys. Chem., 1983, 87, 1984–1990 CrossRef CAS.
- A. A. Dar, G. M. Rather, S. Ghosh and A. R. Das, J. Colloid Interface Sci., 2008, 322, 572–581 CrossRef CAS PubMed.
- R. Ninomiya, K. Matsuoka and Y. Moroi, Biochim. Biophys. Acta, 2003, 1634, 116–125 CrossRef CAS PubMed.
- K. Matsuoka, Y. Kuranaga and Y. Moroi, Biochim. Biophys. Acta, 2002, 1580, 200–214 CrossRef CAS.
- X. Cai, D. J. W. Grant and T. S. Wiedmann, J. Pharm. Sci., 1997, 86, 372 CrossRef CAS PubMed.
- C. Treiner, M. Nortz, C. Vaution and F. Puisieux, J. Colloid Interface Sci., 1988, 125, 261–270 CrossRef CAS.
- C. M. Nguyen, J. F. Scamehorn and S. D. Christian, Colloids Surf., 1988, 30, 335–344 CrossRef CAS.
- C. Treiner, Langmuir, 1987, 3, 729–735 CrossRef CAS.
- G. X. Zhao and X. G. Li, J. Colloid Interface Sci., 1991, 144, 185–190 CrossRef CAS.
- Y. Lin, G. Z. Li and S. X. Hao, NMR study of the solubilization in micelles, in Proceedings of the 92 International Seminar on Surfactants and Detergents, Shanghai, China, 1992, pp. 241–254 Search PubMed.
- Z. Cui and J. P. Canselier, Polym. Int., 2003, 52, 548–552 CrossRef CAS.
- P. Mukerjee, Solubilization in aqueous micelle systems, in Solution Chemistry of Surfactants, ed. K. L. Mittal, Plenum Press, New York, 1979, vol. 1, pp. 153–174 Search PubMed.
- M. A. Chaiko, R. Nagarajan and E. Ruckenstein, J. Colloid Interface Sci., 1984, 99, 168–182 CrossRef CAS.
- R. Nagarajan, M. A. Chaiko and E. Ruckestein, J. Phys. Chem., 1984, 88, 2916–2922 CrossRef CAS.
- R. Nagarajan and E. Ruckenstein, Langmuir, 1991, 7, 2934 CrossRef CAS.
- C. Treiner, M. Nortz and C. Vaution, Langmuir, 1990, 6, 1211 CrossRef CAS.
- C. Treiner, Chem. Soc. Rev., 1994, 23, 349–355 RSC.
- Y. Tokuoka, H. Uchiyama and M. Abe, J. Phys. Chem., 1994, 98, 6167–6171 CrossRef CAS.
- R. Nagarajan, Curr. Opin. Colloid Interface Sci., 1996, 1, 391–401 CrossRef CAS.
- S. Milioto, R. Crisantino and R. D. Lisi, J. Colloid Interface Sci., 1994, 166, 356–362 CrossRef CAS.
- S. Nagadome, Y. Okazaki, S. Lee, Y. Sasaki and G. Sugihara, Langmuir, 2001, 17, 4405–4412 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 rpm to remove the undissolved drug. The concentration of solubilized drug was determined spectrophotometricaly with a Shimadzu spectrophotometer (model UV-1650) following appropriate dilution of an aliquot of the supernatant with the corresponding surfactant concentration. The surfactant concentration was kept the same in both the reference and the measurement cells to eliminate the effect of the surfactant on the UV absorbance. The solubility of CBZ was determined at its characteristic wavelength of 286 nm, at which its extinction coefficient calculated from the calibration curve of the drug in methanol was 1.4335 mM−1 cm−1. Using this extinction coefficient, the solubility of CBZ in water was confirmed to be 6.98 × 10−1 mM, which tallied well with the previously reported value.40 The solubility of NFD was determined at 355 nm and was equal to 3.3 × 10−3 mM in conformity with earlier studies41 using extinction coefficient of 3.28 mM−1 cm−1, determined from the calibration curve of the drug in methanol. The solubilities of CBZ and NFD in the mixture were determined at the above mentioned wavelengths using their respective extinction coefficients. CBZ and NFD absorptions at 286 nm and 355 nm respectively were non-interfering with each other, as depicted by their prototype absorption curves in methanol (Fig. 2a) and in 2 mM (B35 + T20) binary surfactant solution (Fig. 2b). The concentrations of drugs are presented as the mean of three independent measurements corresponding to each surfactant concentration.
400 rpm to remove the undissolved drug. The concentration of solubilized drug was determined spectrophotometricaly with a Shimadzu spectrophotometer (model UV-1650) following appropriate dilution of an aliquot of the supernatant with the corresponding surfactant concentration. The surfactant concentration was kept the same in both the reference and the measurement cells to eliminate the effect of the surfactant on the UV absorbance. The solubility of CBZ was determined at its characteristic wavelength of 286 nm, at which its extinction coefficient calculated from the calibration curve of the drug in methanol was 1.4335 mM−1 cm−1. Using this extinction coefficient, the solubility of CBZ in water was confirmed to be 6.98 × 10−1 mM, which tallied well with the previously reported value.40 The solubility of NFD was determined at 355 nm and was equal to 3.3 × 10−3 mM in conformity with earlier studies41 using extinction coefficient of 3.28 mM−1 cm−1, determined from the calibration curve of the drug in methanol. The solubilities of CBZ and NFD in the mixture were determined at the above mentioned wavelengths using their respective extinction coefficients. CBZ and NFD absorptions at 286 nm and 355 nm respectively were non-interfering with each other, as depicted by their prototype absorption curves in methanol (Fig. 2a) and in 2 mM (B35 + T20) binary surfactant solution (Fig. 2b). The concentrations of drugs are presented as the mean of three independent measurements corresponding to each surfactant concentration.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (40
methanol (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60, v/v), flowing at a rate of 0.5 ml min−1. The instrument was operated at 40 °C. Drugs solubilized in mixed micellar solutions were first centrifuged and then filtered using a 0.2 μm filter paper. 20 μl of the drug solution was injected and the separation was carried out for 45 minutes.
60, v/v), flowing at a rate of 0.5 ml min−1. The instrument was operated at 40 °C. Drugs solubilized in mixed micellar solutions were first centrifuged and then filtered using a 0.2 μm filter paper. 20 μl of the drug solution was injected and the separation was carried out for 45 minutes.






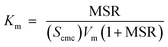


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Km and log
Km and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K(x) of CBZ during single solute solubilization and co-solubilization in (a) C12 series (b) C16 series.
K(x) of CBZ during single solute solubilization and co-solubilization in (a) C12 series (b) C16 series.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Km and log
Km and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K(x) of NFD during single solute solubilization and co-solubilization in (a) C12 series (b) C16 series.
K(x) of NFD during single solute solubilization and co-solubilization in (a) C12 series (b) C16 series.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K(x) = xm
K(x) = xm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K1 + (1 − xm)ln
K1 + (1 − xm)ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K2 + xm(1 − xm)B
K2 + xm(1 − xm)B

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Keq
Keq
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kmixeq
Kmixeq
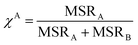
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) γi = ω(1 − χA)2/RT
γi = ω(1 − χA)2/RT






