FeCo/ZnO composites with enhancing microwave absorbing properties: effect of hydrothermal temperature and time
Hualiang Lva,
Guangbin Ji*a,
Min Wanga,
Chaomei Shanga,
Haiqian Zhanga and
Youwei Dub
aCollege of Materials Science and Technology, Nanjing University of Aeronautics and Astronautics, Nanjing 211100, P. R. China.. E-mail: gbji@nuaa.edu.cn; Tel: +86-25-5211-2902
bNational Laboratory of Solid State Microstructures, Nanjing University, Nanjing 210093, P. R. China
First published on 28th October 2014
Abstract
FeCo/ZnO composites were successfully synthesized by a simple one-step hydrothermal process. It is clearly seen that the pencil-like ZnO particles with 3–4 μm in length and 200–300 nm in width are found to grow along the surface of the hexagonal-cone FeCo particles, which form a discontinuous conductive network. Enhanced microwave absorption properties can be obtained for the FeCo/ZnO composites as compared to those of pure FeCo alloy, which is mainly attributed to the better resonance according to an isotropic antenna mechanism. Further investigation confirms that the hydrothermal temperature (T) and time (t) play a key role on the real part of the permittivity values of the FeCo/ZnO composites and indirectly affect the microwave absorbing properties. It is surprising to find out that the composite prepared at 150 °C for 12 hours, exhibited the optimal reflection loss of −31 dB with a 5.5 GHz effective frequency bandwidth.
1. Introduction
In recent years, radar absorber material has drawn intense attention due to its increased use in military and commercial environments.1–3 As we know, a microwave absorber is a type of functional material, which can absorb a radar wave effectively and convert it into thermal energy or other type energies through magnetic loss or dielectric loss. In order to achieve an excellent microwave absorber, a high impedance matching should be obtained, which can let the incident microwave totally propagate into the material without any reflection. After that, the incident microwave can be absorbed by the material itself as much as possible and finally hopefully less incident microwave will be reflected back. In that way, the target, such as an aircraft, cannot be detected by radar. So, the parameter, which is commonly of concern, is the reflection loss (RL) at the surface. Compared to the traditional metallic and ferrite absorbers, semiconductors, as a new kind of radar absorbing material, have attracted great interest in recent years despite of their unclear microwave absorbing mechanism.4,5 For example, He et al. has successfully synthesized complex symmetrical CuS nanostructures, as an absorbent, and the product presented excellent microwave absorbing properties with a minimum RL of −102 dB.6 Meanwhile, one-dimensional MnO2 nanorods were fabricated by Guan et al. and the minimum reflection loss reached −25 dB with a coating thickness of 3 mm.7However, semiconductor materials still suffer from poor impedance matching due to their high permittivity and low permeability. A moderate value between permittivity and permeability should be considered in order to achieve the high microwave absorption properties. Hence, magnetic-semiconductor composites have been proposed to be a new candidate of microwave absorbing material, which can take advantage of both a unique permittivity and strong magnetic properties. Recently, some efforts have been made on Fe3O4 based materials. Research on Fe3O4@TiO2 has proven that the product exhibits higher microwave absorbing abilities than pure Fe3O4, with the RL value of the Fe3O4@TiO2 reaching −29.7 dB, while the pure Fe3O4 reached only −10.7 dB.8 Similarly, the same conclusion has been confirmed by another study, where the RL value for Fe3O4/ZnO was −22.96 dB and for pure Fe3O4 it was just about −3.3 dB.9 However, according to the following equation,10
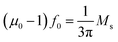 | (1) |
, it can be clearly seen that a high value of Ms led to a high value of μ0. It is well known that Ms of a magnetic metal oxide is still too low to get a high μ0 value. Thus, a material with high saturation magnetization is required for producing magnetic-semiconductor composites with a better microwave absorption performance. Among all the candidates, FeCo alloys can be used as an ideal candidate because of their high saturation magnetization, high magnetic loss ability as well as high Curie temperature (Tc).11,12 So, in this study, an FeCo/ZnO composite has been prepared by a simple one-step hydrothermal method. Furthermore, the reaction temperature and the time have an important effect on the value of the minimum reflection loss and the effective frequency width.
2. Experimental
2.1 Hydrothermal synthesis of FeCo/ZnO composites
All of the chemical reagents used in this study were analytically pure and were used without further purification. The FeCo/ZnO composites were successfully synthesized by a simple hydrothermal approach. Briefly, 4 mmol of FeSO4 and 4 mmol CoCl2 were first dissolved in 50 mL distilled water under mechanical stirring. Then, 20 mL of 3 M NaOH was added into the mixing solution. Secondly, 5 mL of hydrazine hydrate (85%) was added to the solution. After that, the mixing solution was transferred into a Teflon-lined stainless steel autoclave (containing 4 mmol of ZnCl2), and subsequently sealed and heated at 150 °C for 12 h in an oven. Finally, the resulting product was collected by magnetic separation and washed with absolute ethanol and distilled water for several times and then dried in a vacuum oven at 60 °C for 12 h. In addition, the pure FeCo and FeCo/ZnO composites were also synthesized using this method only by varying the reaction temperature (130 °C/140 °C)/12 h or time (15 h, 20 h)/150 °C.2.2 Characterizations
Powder X-ray diffraction (XRD) pattern measurements were performed on a Bruker D8 ADVANCE X-ray diffractometer using Cu Kα radiation (λ = 0.154178 nm) with 40 kV scanning voltage, 40 mA scanning current and a scanning range from 30° to 90°. A Hitachi S4800 type scanning electron microscope (operating at an acceleration voltage of 3.0 kV and equipped with energy dispersive X-ray spectroscopy) was used to observe the morphology and size of the FeCo/ZnO composite. The magnetic performances, including the saturation magnetization and the coercive force, were tested via a vibrating sample magnetometer (VSM, Lakeshore, Model 7400 series). The S parameter, including S11, S12, S21 and S22, was measured by an Agilent PNA N5224A vector network analyzer using the coaxial-line method, for which the samples were prepared by homogeneously mixing the paraffin wax with a certain amount of sample and pressing them into toroidal-shaped samples (Φout: 7.0 mm, Φin: 3.04 mm). After that, a software, which has been installed in Agilent PNA, calculated the ε′, ε′′, μ′, μ′′ values. Finally, the RL value with different thicknesses can be calculated by using the following formulae:13–17
 | (2) |
RL (dB) = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log|(Zin − Z0)/(Zin + Z0)| log|(Zin − Z0)/(Zin + Z0)|
| (3) |
3. Results and discussion
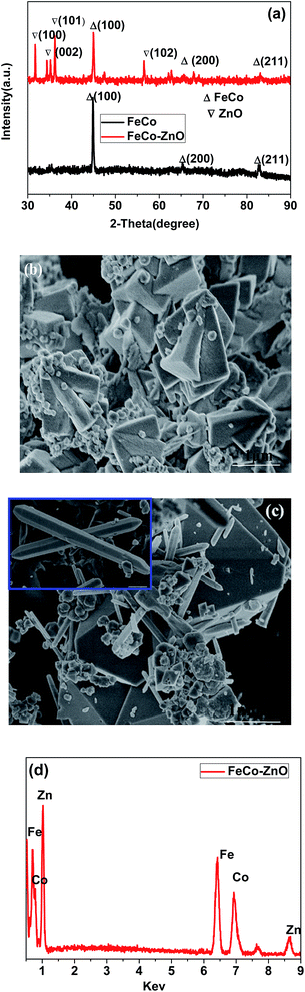
The phase structure of the FeCo/ZnO composite and pure FeCo were characterized by XRD. As displayed by the black line in Fig. 1a, the three major diffraction peaks at 44.6°, 65.4° and 82.7° can be easily indexed to (110), (200) and (211), respectively. Moreover, the sharpness of the peaks suggests a high crystalline quality. Compared to the pure FeCo, these strong peaks at around 31.6°, 34.2°, 36.1° can be assigned to a pure hexagonal wurtzite phase of ZnO (JCPDS 36-1451). For comparison, the morphology, structure, and size of the pure FeCo and FeCo composites were also characterized by FESEM. As displayed in Fig. 1b, pure FeCo has a hexagonal cone structure with a smooth surface, and the size is mainly distributed around 1.5 μm. In Fig. 1c, it is clearly shown that the pencil-like ZnO particles with 3–4 μm in length and 200–300 nm in width are found to grow along the surface of the hexagonal-cone FeCo particles, which form a discontinuous conductive network. The EDX analysis of the FeCo/ZnO composites further confirms the presence of Fe, Co and Zn (Fig. 1d).Subsequently, the magnetic properties of pure FeCo and FeCo/ZnO (150 °C/12 h) were measured between −10 kOe and 10 kOe, shown in Fig. 2a. It can be clearly seen that pure FeCo has excellent soft magnetic properties and the value of magnetization reaches 140 emu g−1. As far as the FeCo/ZnO composite is concerned, the value of magnetization sharply decreases to 60 emu g−1, due to the non-magnetic ZnO. The reflection loss of FeCo, ZnO and the FeCo/ZnO composites, with the same coating thickness of 1.5 mm, is presented in Fig. 2b. Compared to FeCo and ZnO, the FeCo/ZnO composites have obvious microwave absorbing properties. The minimum reflection loss of FeCo/ZnO can reach −31 dB, while for pure FeCo and ZnO it is only −22 dB and −7 dB, respectively. Meanwhile, the frequency width below −10 dB can reach up to 5.5 GHz for FeCo/ZnO, while for pure FeCo it has only 2.5 GHz.
 | ||
| Fig. 2 M-H loops, the reflection loss, the dielectric loss, and ε′ − ε′′ curves of FeCo and the FeCo/ZnO composites. | ||
In order to explore the mechanism of the enhancing microwave absorbing properties, the dielectric tangent loss of FeCo, ZnO and FeCo/ZnO also has been calculated, as shown in Fig. 2c. Obviously, the FeCo/ZnO composites have a stronger dielectric loss behavior than pure FeCo and ZnO. It is well-known that at a high frequency range, the enhanced dielectric loss behavior is mainly caused by resonance behavior. Consistent with Fig. 2d, it is noted that the FeCo/ZnO composites exhibit a remarkable resonance behavior, appearing at 8–10 GHz and 12–18 GHz.
In fact, the enhancement of the dielectric loss can be explained by the isotropic antenna mechanism.18,19 It is noted that the rod-like shape of ZnO is more apt to produce a vibrating microcurrent under the external magnetic field. Through the external magnetic field, the microcurrent, transferred in the discontinuous conductive networks, is attributed to electromagnetic attenuation. However, the contact between ZnO and FeCo also can cause the polarization lag, which favours dielectric loss. Except for the stronger dielectric loss, FeCo with a high permeability and magnetic loss has contributed to the impedance match and the attenuation ability.
It is also found that the temperature and time have a significant influence on the magnetic and microwave absorbing properties. Fig. 3 shows the magnetization of FeCo/ZnO prepared under various temperatures and times. From Fig. 3a, we can discover that the value of the magnetization increases from 65 to 85 emu g−1 with the time increasing from 12 to 15 hours and then decreases to 75 emu g−1 after 20 hours. When varying the temperature, as shown in Fig. 3b, the variation tendency of the magnetization is similar to the changing of the time. The highest magnetization is 75 emu g−1 at 140 °C and the lowest magnetization is 61 emu g−1 at 150 °C. It can be concluded that after too much time or at a too high temperature for the hydrothermal reaction, parts of the Fe or Co element has been slightly oxidized and formed weak or non-magnetic α-Fe2O3, which resulted in a decreased magnetization.
 | ||
| Fig. 3 The M-H loops of the FeCo/ZnO composites under different conditions: (a) reaction time; (b) reaction temperature. | ||
In addition, the microwave absorbing properties were also effected by the various temperatures and times during the hydrothermal process. Fig. 4a displays the reflection loss of FeCo/ZnO after varying reaction times from 12 to 20 hours, with the same matching thickness of 1.5 mm. As we know, the minimum reflection loss and a frequency width less than −10 dB (corresponding to 90% of microwave absorbed) should be taken into consideration to obtain an excellent microwave absorbing material. Thus, it is noted that the FeCo/ZnO composites obtained at 12 hours exhibit the best microwave properties, although their minimum reflection loss is −31 dB, which is a little smaller than for the sample obtained after 15 hours. But, the FeCo/ZnO composites after 12 hours have a wider frequency width than any other sample, for which the reflection loss exceeding −10 dB can reach 5.5 GHz. The real part of the permittivity can explain the law of the changing microwave absorbing properties well (Fig. 4b). It is well known that a high ε′ value is harmful to impedance matching, which in turn decreases the microwave absorbing properties. From Fig. 4b we find that all the samples of the FeCo/ZnO composites obtained after different times exhibit a broad resonance peak located between 8 and 15 GHz. In the frequency region (2–13 GHz), the sample prepared after 12 hours shows the lowest ε′ value, corresponding to a value increasing from 11 to 12.5, which is due to impedance matching and in turn improves the microwave absorbing properties.
 | ||
| Fig. 4 The reflection loss (a) and the real part of the permittivity (b) of the FeCo/ZnO composites with the same coating thickness of 1.5 mm at different reaction times. | ||
Fig. 5a presents the reflection loss of the FeCo/ZnO composites prepared at different temperatures. Compared to the samples synthesized at 130 or 140 °C, both the minimum value and the effective frequency width of the FeCo/ZnO composites prepared at 150 °C during the hydrothermal process have significantly improved, which the minimum value of the reflection loss being −31 dB. The minimum reflection loss is −18 dB and −17 dB, and the effective frequency width is only 3.3 GHz and 2.7 GHz at a hydrothermal temperature of 130 °C and 140 °C, respectively. The ε′ values have also been used for further explaining the changing microwave absorbing properties (Fig. 5b). From 2 to 11 GHz and 14.5 to 18 GHz, the sample prepared at 150 °C has the lowest ε′ value. At the same time, the sample prepared at 140 °C has the largest ε′ value during most of 2–18 GHz. Thus, the sample prepared at 150 °C has the best microwave absorbing properties while the sample prepared at 140 °C has the poorest microwave absorbing ability.
 | ||
| Fig. 5 The reflection loss (a) and the real part of the permittivity (b) of the FeCo/ZnO composites at different reaction temperatures. | ||
4. Conclusion
In summary, FeCo/ZnO composites have been synthesized by a simple one-step hydrothermal method. In comparison with pure FeCo and ZnO, the microwave absorbing properties of the FeCo composites were significantly improved. Moreover, the temperature and time during the reaction also have an influence on the microwave absorbing properties. The FeCo/ZnO composite, synthesized at 150 °C for 12 hours, exhibits the best microwave absorbing properties, with the minimum reflection loss being −31 dB and the effective frequency bandwidth reaching 5.5 GHz, while the coating thickness was just 1.5 mm.Acknowledgements
The financial supports by the Aeronautics Science Foundation of China (2014ZF52072), the National Natural Science Foundation of China (51172109 and 11475086) and the Fundamental Research Funds for the Central Universities (no: NS2014057) are gratefully acknowledged.Notes and references
- A. M. Wang, W. Wang, C. Long, W. Li, J. G. Guan, H. S. Gu and G. X. Xu, J. Mater. Chem. C, 2014, 2, 3769–3776 RSC.
- H. L. Lv, G. B. Ji, M. Wang, C. M. Shang, H. Q. Zhang and Y. W. Du, J. Alloys Compd., 2014, 615, 1037–1042 CrossRef PubMed.
- S. Maiti, N. K. Shrivastava, S. Suin and B. B. Khatua, ACS Appl. Mater. Interfaces, 2013, 5, 4712–4724 CAS.
- M. Zhou, X. Zhang, J. M. Wei, S. L. Zhao, L. Wang and B. X. Feng, J. Phys. Chem. C, 2011, 115, 1398–1402 CAS.
- Q. Hu, G. X. Tong, W. H. Wu, F. T. Liu, H. S. Qian and D. Y. Hong, CrystEngComm, 2013, 15, 1314–1323 RSC.
- S. He, G. S. Wang, C. Lu, J. Liu, B. Wen, H. Liu, L. Guo and M. S. Cao, J. Mater. Chem. A, 2013, 1, 4685–4692 CAS.
- H. T. Guan, J. B. Xie, G. Chen and Y. D. Wang, Mater. Chem. Phys., 2014, 143, 1061–1068 CrossRef CAS PubMed.
- J. W. Liu, J. J. Xu, R. C. Che, H. J. Chen, M. M. Liu and Z. W. Liu, Chem.–Eur. J., 2013, 19, 6746–6752 CrossRef CAS PubMed.
- Z. J. Wang, L. N. Wu, J. G. Zhou, B. Z. Shen and Z. H. Jiang, RSC Adv., 2013, 3, 3309–3315 RSC.
- C. L. Zhu, M. L. Zhang, Y. J. Qiao, G. Xiao, F. Zhang and Y. J. Chen, J. Phys. Chem. C, 2010, 114, 16229–16235 CAS.
- P. Sirvent, E. Berganza, A. M. Aragon, A. Bollero, A. Moure, M. G. Hernandez, P. Marin, J. F. Fernandez and A. Quesada, J. Appl. Phys., 2014, 115, 17B505–17B507 CrossRef PubMed.
- N. Poudyal, C. B. Rong and J. P. Liu, J. Appl. Phys., 2011, 109, 07B526–07B528 CrossRef PubMed.
- S. Maiti, N. K. Shrivastava, S. Suin and B. B. Khatua, ACS Appl. Mater. Interfaces, 2013, 5, 4712–4724 CAS.
- A. Ohlan, K. Singh, A. Chandra and S. K. Dhawan, ACS Appl. Mater. Interfaces, 2010, 2, 927–933 CAS.
- J. Zheng, H. L. Lv, X. H. Lin, G. B. Ji, X. G. Li and Y. W. Du, J. Alloys Compd., 2014, 589, 174–181 CrossRef CAS PubMed.
- X. G. Li, G. B. Ji, H. L. Lv, M. Wang and Y. W. Du, J. Magn. Magn. Mater., 2014, 355, 65–69 CrossRef CAS PubMed.
- M. Zong, Y. Huang, Y. Zhao, X. Sun, C. H. Qu, D. D. Luo and J. B. Zheng, RSC Adv., 2013, 3, 23638–23648 RSC.
- R. F. Zhuo, H. T. Feng, J. T. Chen, D. Yan, J. J. Feng, H. J. Li, B. S. Geng, S. Cheng, X. Y. Xu and P. X. Yan, J. Phys. Chem. C, 2008, 112, 11767–11775 CAS.
- R. F. Zhuo, L. Qiao, H. T. Feng, J. T. Chen and D. Yan, J. Appl. Phys., 2008, 104, 094101–094105 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2014 |