Facile fabrication of red phosphorus/TiO2 composites for lithium ion batteries
Abstract
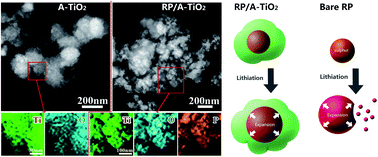
Red phosphorus (RP) is an attractive anode material with an ultrahigh specific capacity of 2596 mA h g−1. However, its rapid capacity decay attributed to the volume expansion during the lithiation process presents a noteworthy technical challenge. Meanwhile, titanium oxide (TiO2) is a good candidate for lithium ion batteries owing to its high safety and outstanding stability, but it is restricted by the low capacity of 167 mA h g−1 at room temperature. Inspired by reinforced concrete structures, we fabricate an RP built-in amorphous TiO2 (A-TiO2) composite in consideration of achieving complementary effects. Herein, A-TiO2 could act as “concrete” to prevent RP from escaping the electrode. While RP plays the role of “steel”, which could improve the electrochemical capacity of the composite. As a result, the RP/A-TiO2 composite demonstrates an enhanced cycling capacity of 369 mA h g−1 over 100 cycles as well as an acceptable rate capacity of 202 mA h g−1 at the current density of 1 A g−1. This designed unique reinforced concrete structure may provide a novel strategy to fabricate high electrochemical performance anodic materials for advanced lithium ion batteries.

 Please wait while we load your content...
Please wait while we load your content...